Euphorbia Helioscopia Inhibits The LPS-Induced Pro-Inflammatory Response in RAW 264.7 Cells Via The NF-Κb and MAPK Pathway
Eun-Jin Yang, Juyeong Lee, Duk Soo Kim, Nam Ho Lee, and Chang-Gu Hyun*
Cosmetic Sciences Center, Department of Chemistry and Cosmetics, Jeju National University, Jeju 63243, Korea. Corresponding Author E-mail: cghyun@jejunu.ac.kr
DOI : http://dx.doi.org/10.13005/ojc/320605
Previous studies have characterized the total polyphenolic contents, antioxidant activity, tyrosinase, elastase, and NO production of halophytes. Halophytes are distributed among many countries; however, it has not been properly utilized. In this study, we investigated the inhibitory effect of halophyte Euphorbia helioscopia (E.H.) on the LPS-induced inflammatory response in RAW 264.7 macrophages by MTT assay, NO assay, ELISA, and western blot analysis. Our results demonstrate that E.H. reduced LPS-induced NO and PGE2 in addition to pro-inflammatory mediators, IL-6, IL-1β and TNF-α. Furthermore, E.H. inhibited LPS-induced activation of NF-κB, via IκBα degradation and phosphorylation of JNK and ERK. Our data suggest that the E.H. anti-inflammatory effect is a result of inhibition of LPS-induced NO, PGE2, IL-6, IL-1β, and TNF-α production via downregulation of the NF-κB and MAPK pathway.
KEYWORDS:Euphorbia helioscopia; halophyte; inflammation; MAPK; NF-κB
Download this article as:| Copy the following to cite this article: Yang E, Lee J, Kim D. S, Lee N. H, Hyun C. Euphorbia Helioscopia Inhibits The LPS-Induced Pro-Inflammatory Response in RAW 264.7 Cells Via The NF-Κb and MAPK Pathway. Orient J Chem 2016;32(6). |
| Copy the following to cite this URL: Yang E, Lee J, Kim D. S, Lee N. H, Hyun C. Euphorbia Helioscopia Inhibits The LPS-Induced Pro-Inflammatory Response in RAW 264.7 Cells Via The NF-Κb and MAPK Pathway. Orient J Chem 2016;32(6). Available from: http://www.orientjchem.org/?p=25102 |
Introduction
Lipopolysaccharide (LPS)-induced activation of murine RAW 264.7 cells results in the production of pro-inflammatory cytokines including PGE2, IL-6, TNF-α, and IL-1β. Cytokine production is regulated by cellular signaling events, such as the NF-κB and MAPK pathway, mediating inflammation, and the immune response to infection (1-5).
About 1500 species of salt-tolerant halophytes are distributed in the costal wetland of river estuaries, saltpans, and reclaimed land. Moreover, about 40-100 species are distributed in Korea. Halophytes are rich in natural minerals owing to their ability to grow in the soil of seawater (6, 7).
We recently characterized the total polyphenolic content, antioxidant activity, tyrosinase, elastase, and nitric oxide (NO) production of 21 halophyte species in vitro (8). Among them, Euphorbia helioscopia (E.H)., used as traditional Chinese medicine, and its extracts were found to inhibit various carcinomas, in addition to having antifungal and antibacterial activity (9, 10). However, there have been no reports on its anti-inflammatory activity. Therefore, we evaluated the effect of E.H. on NO, iNOS, and pro-inflammatory cytokine production. Further, to establish the anti-inflammatory mechanism for E.H., we examined the NF-κB and MAPK signaling pathways in the murine RAW 264.7 cells.
Materials and Methods
Cells and reagents
A RAW 264.7 mouse macrophage cell line was obtained from the Korean Cell Line Bank (Seoul, Korea). Cells were cultured in Dulbecco’s Modified Eagle’s medium (DMEM containing 2 mM glutamine, 10 mM HEPES, 100 µg/mL penicillin-streptomycin, and 10 % fetal bovine serum (FBS) at 37 ℃ under 5 % CO2in a humidified incubator. Lipopolysaccharide (LPS) was purchased from Sigma Aldrich (St. Louis, MO, USA). For ELISA, IL-6, PGE2, IL-1β and TNF-α were obtained from R&D Systems (St. Louis, MO, USA). Anti-iNOS antibody and anti-COX-2 antibody were purchased from BD Bioscience (San Diego, CA, USA). All other antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA).
MTT cell viability assay
RAW 264.7 macrophage cells were cultured in 96-well plates for 18 h, followed by treatment with various concentrations (50 to 200 µg/mL) of the E. helioscopia for 24 h. Cell viability was then perforemed by 3-(4,5-dimethylthiazol-2yl)-,5-diphenyltetrazolium bromide (MTT) assay (11, 12). Formazan crystals were dissolved in dimethyl sulphoxide (DMSO) and percent cytotoxicity was determined relative to the untreated control group.
Nitric oxide assay
Nitrite levels was used as an indicator of NO production in the cell culture medium by the Griess reagent (1% sulfanilamide, 0.1% naphthylethylenediaminedihydrochloride, and 2.5% phosphoric acid) (13, 14). Specifically, culture supernatant from each well was mixed with equal volume Griess reagent for 15 min, and optical density was determined at 540 nm. Percent NO production was determined relative to the control LPS only treated group.
ELISA determination of cytokines
Cells (1 × 106) were cultured for 24 h in the presence or absence of 1 µg/mL LPS and various concentrations of E. helioscopia(50, 100, 200 µg/mL). Cell supernatants were harvested and centrifuged at 4,500 rpm for 4 min to remove dead cells. Cytokine production percent was determined relative to the control LPS only treated group.
Western blot analysis
Cells were washed twice with cold PBS, followed by lysis in RIPA lysis buffer on ice for 20 min. Cell lysates were centrifuged at 12,000 rpm for 20 min at 4 ℃ and protein concentration was determined using the BCA method. Cell lysates were separated by 8% Bis-Tris gel and transferred with the iBlot 7-minute blotting system to iBlot Gel transfer stacks (ThermoFisher Scientific, USA). The membrane was blocked using 5% skim milk for 1 h at room temperature, followed by an overnight incubation at 4 ℃ 2 h with 1:1000 dilution primary antibody. Following four washes, the membranes were incubated with 1:5,000 dilution of horseradish peroxidase-conjugated secondary antibody for 30 min at room temperature. Membrane proteins were detected using the ECL western blotting detection substrate.
Data analysis
All data were expressed as the mean ± standard deviation (S.D) of at least 3 replicates. The Student’s t-test and one-way ANOVA were used for statistical analysis, and *P<0.005, **P<0.001 was considered significant.
Results
Effect of E. helioscopiaon the cell viability and NO production of RAW 264.7 cells
We investigated the effect of E.H. on LPS-treated RAW 264.7 cells after 24 h by cell viability and NO production. We found that E.H. was not cytotoxic to LPS-treated RAW 264.7 cells at concentrations up to 200 µg/mL by MTT assay (Figure 1). To investigate the anti-inflammatory effects of E.H., we analyzed LPS-induced NO production in E.H.-treated cells. Cells were treated with 1 µg/mL of LPS and various concentration of E.H.for 24 h and NO levels in the media were determined. We found that E.H. reduced NO production in LPS-treated cells in dose dependent manner (Figure 1). We utilized western blot analysis to determine if the inhibitory effect of E.H. on NO production was due to a reduction in iNOS expression. As expected, LPS markedly induced iNOS protein levels; however, treatment with E.H. inhibited iNOS expression in LPS-treated cells suggesting that the inhibitory effect of E.H. on LPS-induced NO production was due to the suppression of iNOS (Figure 2).
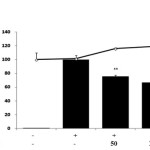 |
Figure 1: Effect of Euphorbiahelioscopia on nitric oxide production in LPS-treated RAW 264.7 cells.Cells were treated with 1 µg/mL of LPS or with LPS plus various concentrations (50 to 200 µg/mL) of E. helioscopia for 24 h. Nitric oxide (NO) production was determined by the Griess reagent method. Cell viability was determined from the 24 h culture of cells treated with LPS (1 µg/mL) in the presence of E. helioscopia. The data represent the mean ± SD of triplicate experiments. *p <0.005, **p<0.001 versus LPS alone.
|
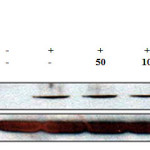 |
Figure 2: Effect of Euphorbiahelioscopiaon the protein level of iNOS in LPS-treated RAW 264.7 cells. RAW 264.7 cells (6.0 × 105 cell/mL) treated with LPS (1 µg/mL) in the presence of E. helioscopia(50 to 200 µg/mL) for 24 h. Whole-cell lysates (30 µg) were prepared, protein was subjected to 8% SDS-PAGE, and expression of iNOS and control β-actin was determined by western blotting. |
Effect of E. helioscopiaon the LPS-induced PGE2 production
PGE2 is a pro-inflammatory mediator implicated in many different inflammatory diseases. To analyze the inhibitory effect of E.H. on the production of PGE2, we utilized ELISA. We found that E.H. significantly reduced PGE2 production by 90 % in the LPS stimulated RAW 264.7 cells (Figure 3).
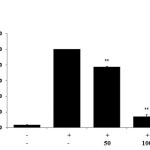 |
Figure 3: Effect of Euphorbiahelioscopiaon PGE2 production in LPS-treated RAW 264.7 cells.Cells were treated with 1 µg/mL of LPS or with LPS plus various concentrations (50 to 200 µg/mL)of E. helioscopiafor 24 h. PGE2 production was determined by ELISA. The data represent the mean ± SD of triplicate experiments. *p <0.005, **p<0.001 versus LPS alone. IC50= 75.4 µg/mL. |
Effect of E. helioscopiaon the LPS-induced pro-inflammatory cytokine production
To determine if E.H. reduces LPS-induced pro-inflammatory cytokines IL-6, IL-1β and TNF-α production, we utilized ELISA. We found that E.H. strongly inhibited IL-6, IL-1β and TNF-α production in the LPS-treated RAW 264.7 cells by 63.6%, 58.9% and 46.6 %, respectively (Figure 4).
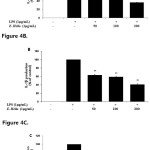 |
Figure 4: Effect of Euphorbiahelioscopiaon IL-6, IL-1β, and TNF-α production in LPS-treated RAW 264.7 cells.Cells were stimulated with 1 µg/ml of LPS or with LPS plus various concentrations (50, 100, 200 µg/mL) of E. helioscopiafor 24 h. IL-6 (A), IL-1β (B), and TNF-α (C) productions were determined by ELISA. The data represent the mean ± SD of triplicate experiments. *p <0.005, **p<0.001 versus LPS alone. Click here to View figure |
Effect of E. helioscopiaon the LPS-induced NF-κB activationand IκBα degradation
Activation of NF-κB is critical for the production of iNOS, PGE2, IL-6, IL-1β, and TNF-α in LPS-treated murine RAW 264.7 macrophages. IκBα phosphorylation and degradation is required for NF-κB activation (15). To determine if E.H.-induced NF-κB inhibition is due to IκBα phosphorylation and degradation, we analyzed cytoplasmic levels of NF-κB. As expected, LPS treatment led to a reduction of IκBα levels; however, treatment with E.H. weakly blocked the LPS-induced IκBα degradation. Further, we found that E.H. significantly suppressed LPS-induced phosphorylation of NF-kB in cytoplasm (Figure 5).
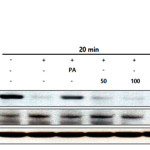 |
Figure 5: Effect of halophyte Euphorbiahelioscopia on the degradation of IκBα and activation of NF-κB p-p65 in LPS-treated RAW 264.7 cells. RAW 264.7 cells (1.0×106 cells/mL) were treated with LPS (1 µg/mL) in the presence of E. helioscopia (50 to 200 µg/mL) or palmitate (20 µM) for 20 minutes. Whole cell lysates (30 µg) were prepared, protein was subjected to 8% SDS-PAGE, and expression of IκBα, P-p65 and control β-actin was determined by western blotting. |
Effect of E. helioscopiaon the LPS-induced JNK and ERK phosphorylation
The MAPK pathway plays an important role in the activation of NF-κB (15, 16). To investigate whether the E.H.-induced NF-κB inhibition is mediated through the MAPK pathway, we analyzed LPS-induced p-JNK and p-ERK kinase expression in E.H.-treated RAW 264.7 cells. E.H. suppressed LPS-induced activation of p-JNK and p-ERK while the expression of non-phosphorylated JNK and ERK was unaffected by both LPS or LPS plus E.H treatment (Figure 6).
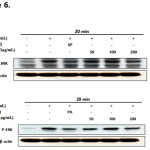 |
Figure 6: Effect of Euphorbiahelioscopia on the phosphorylation of JNK (A) and ERK(B) in LPS-treated RAW 264.7 cells.RAW 264.7 cells (1.0×106 cells/mL) were treated with LPS (1 µg/mL) in the presence of E. helioscopia (50 to 200 µg/mL) or inhibitors (40 µM) for 20 minutes. Whole cell lysates (30 µg) were prepared, protein was subjected to 10% SDS-PAGE, and expression of p-JNK, p-ERK and control β-actin was determined by western blotting. SP600125 (SP): JNK inhibitor, PD98059 (PD): ERK inhibitor.
|
Conclusion
Both NO and PGE2, produced by activated macrophages, play critical roles in inflammatory diseases. In the present study, we found that E.H. inhibits NO and PGE2 production, in addition to iNOS expression, in dose dependent manner. The inhibition of NO, PGE2 and iNOS was not due to E.H.-induced cytotoxicity as cell viability was unaffected when assessed by MTT assay.
Pro-inflammatory cytokines, such as, IL-6, IL-1β and TNF-α, pro-inflammatory are associated with the immunopathology of inflammatory diseases such as rheumatism and various autoimmune diseases. In this study, we found that E.H. significantly inhibited IL-6, IL-1β and TNF-α production. NF-κB plays a critical role in regulating NO, PGE2 and pro-inflammatory cytokines. To determine if E.H.-induced inhibition of NO, PGE2, IL-6, IL-1β, and TNF-α result from the inhibition of NF-κB, we examined phosphorylation in the NF-κB and MAPK pathways. We found that the activity of p65, JNK and ERK was inhibited by E.H. in LPS-treated RAW 264.7 cells.
These data suggest that the E.H.-induced phosphorylation in the MAPK pathway may contribute to its inhibitory effect on NF-κB in E.H.-treated RAW 264.7 cells. Further, E.H. may block LPS-induced IκBα degradation, inhibiting NF-κB activation.
In summary, our findings suggest that E.H. is a potent inhibitor of LPS-induced NO, PGE2, IL-6, IL-1β, and TNF-α production in RAW 264.7 cells. Moreover, the inhibitory effect of E.H. was found to be associated with NF-κB inactivation via blockade of IκBα phosphorylation and degradation.
This study is the first to provide evidence that E. helioscopiainhibits NO, PGE2 and pro-inflammatory mediators in LPS-treated RAW 264.7 cells via the blockade of the NF-κB and MAPK pathways.
Acknowledgements
This research was supported by the 2016 Scientific Promotion Program funded by Jeju National University.
References
- Fang, H.; Pengal, R.A.; Cao, X.; Ganesan, L.P.; Wewers, M.D.; Marsh, C.B.; Tridandapani, S.; J.Immunol.2004, 173, 360-366.
CrossRef - Agrawal, D.K.; Shao, Z.; Curr. Allergy Asthma Rep.2010, 10, 39-48.
CrossRef - Kim, M.J.; Yang, K.W.; Yang, E.J.; Kim, S.S.; Park, K.J.; An, H.J.; Choi, Y.H.; Lee, N.H.; Hyun, C.G.; Orient. J. Chem. 2015, 31, 1915-1922.
CrossRef - Yang, E.J.; Hyun, G.H.; Kim, H., Kim, M.J.; Lee, N.H.; Hyun, C.G.; Orient. J. Chem. 2016, 32, 29-35.
CrossRef - Hyun, E.A.; Kang, J.S.; Yang, K.W.; Kim, S.Y.; Kim, S.C.; Lee, W.J.; Lee, N.H.; Hyun, C.G.; Orient. J. Chem. 2016, 32, 1749-1757.
CrossRef - Jeong, J.H.; Kim, T.K.; Choi W.Y.; Kim, S.R.; Development Administration RDA Interrobang, 2014, 1-15 (Korean)
- Kong, C.S. Prev. Nutr. Food Sci.2014, 19, 261-267
CrossRef - Yang, E.J.; Hyun, J.M.; Lee, N.H.; Hyun, C.G.; Int. J.ChemTech Res.2016, 9, 541-547.
- Cheng, J.; Han, W.; Wang, Z.; Shao, Y.; Wang, Y.;, Zhang, Y.; Li, Z.; Xu, X.; Zhang, Y.; BioMed Res. Int.2015, 2015, 601015.
- Wang, Z.Y.; Liu, H.P.; Zhang, Y.C.; Guo, L.Q.; Li, Z.X.; Shi, X.F.; Anat. Rec.(Hoboken), 2012, 295, 223-233.
CrossRef - Gerlier, D.; Thomasser, N.; J.ImmunolMethods.1986, 94, 57-63.
CrossRef - Liu, Y. Prog.Neuropsychopharmacol. Biol. Psychiatry.1996, 23, 377-395.
CrossRef - Snell, J.C.; Colton, C.A.; Chernvshev, O.N.; Gilgert, D.L.; Free Radic. Biol. Med, 1996, 20, 361-363.
CrossRef - Woo, E.R.; Lee, J.Y.; Cho, I.J.; Kim, S.G.; Kang, K.W.; Pharmacol.Res.2005, 51, 539-546.
CrossRef - Guha, M.; Mackman, N.; Cell Signal.2001, 13, 85-94.
CrossRef - Irie, T.; Muta, T.; Takeshige, K.; FEBS Lett.2000, 467, 160-164.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









