Development and Optimization of Producing 3,3',4,4'-Benzophenonetetracarboxylic Dianhydride
Anton Sergeyevich Yegorov*, Alyona Igorevna Wozniak, Vitaly Sergeyevich Ivanov, Elena Aleksandrovna Averina and Olga Anatolevna Zhdanovich
Federal State Unitary Enterprise «State Scientific Research Institute of Chemical Reagents and High Purity Chemical Substances»(FSUE “IREA”) 107076, Bogorodsky val, 3. Moscow. Russia.
Corresponding Author E-mail: egorov@irea.org.ru
DOI : http://dx.doi.org/10.13005/ojc/320627
This paper includes a series of experiments conducted in order to develop a laboratory method for producing 3,3', 4,4'-benzophenonetetracarboxylic dianhydride and selection of the optimum conditions, allowing to obtain the greatest yield.3,3',4,4'-benzophenonetetracarboxylic dianhydrideis used for the preparation of polyimideswith high thermal stability, chemical resistance as well as high strength and high Young’s modulus. Benzophenonetetracarboxylicdianhydridewas prepared by multistep route:Friedel-Crafts alkylation of o-xylene to obtain 3,3', 4,4'-tetramethylbenzophenonefollowed by liquid phase oxidation to benzophenonetetracarboxylic acid and dehydration.
KEYWORDS:3,3',4,4'-benzophenonetetracarboxylic dianhydride; dianhydrides; polyimides; alkylation; heat resistant polymers; monomer
Download this article as:| Copy the following to cite this article: Yegorov A. S, Wozniak A. I, Ivanov V. S, Averina E. A, Zhdanovich O. A. Development and Optimization of Producing 3,3',4,4'-Benzophenonetetracarboxylic Dianhydride. Orient J Chem 2016;32(6). |
| Copy the following to cite this URL: Yegorov A. S, Wozniak A. I, Ivanov V. S, Averina E. A, Zhdanovich O. A. Development and Optimization of Producing 3,3',4,4'-Benzophenonetetracarboxylic Dianhydride. Orient J Chem 2016;32(6). Available from: http://www.orientjchem.org/?p=25420 |
Introduction
It is necessary to improve polymers due to the extensive use of polymeric materials in terms of maintaining their properties during heating, increase of the heat, thermal and radiation stability of the polymers and polymeric materials.Heat resistance of polymers and polymeric materials determines their shape stability, including deformation stability when heated.
Polyimides are the class of thermally stable polymers, generally with rigid aromatic backbone chains. The most common method for producing aromatic polyimide is the reaction of dianhydrides of aromatic tetra carboxylic acids with aromatic diamines. This reaction is carried out both by a two-stage or a single-stage method [1]. The most common is the two-stage method. A soluble prepolymer is obtained at the first stage, of which films, fibers, coatings and other products can be formed. This reaction consists in the acylation of diamine with tetracarboxylic dianhydride in a polar solvent to form a polyamic acid (PAA) according to the equation shown in Figure 1.
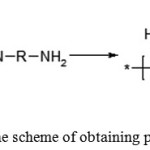 |
Figure 1: The scheme of obtaining polyamide acid |
The second stage of the reaction consists in dehydrocyclization of polyamic acid (PAA) (imidization) (see Figure 2) to form the end- product the aromatic polyimide (PI) – and carried out by thermal or chemical methods 2.
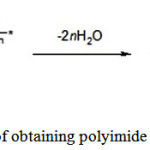 |
Figure 2: Scheme of obtaining polyimide from polyamide acid |
In this paper, 3,3′,4,4′-benzophenonetetracarboxylic dianhydride (Figure 3) has been selected as a target, which furthercan be used to obtain the polyimide. The dianhydride is a powder of white or slightly grayish color, which isresistant to air moisture at room temperature; melting point is 225-228ºC [3].
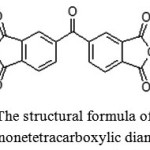 |
Figure 3: The structural formula of 3,3 ‘, 4,4’-benzophenonetetracarboxylic dianhydride |
The literature contains information about methods for preparation the appropriate dianhydride by an hydridization of corresponding tetra-carboxylic acid, obtained by oxidation of 3,3′,4,4′-tetra alkyl benzophenone (mostly 3,3′, 4,4′-tetramethylbenzophenone), as shown in Figure 4.
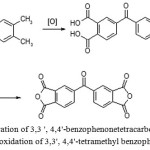 |
Figure 4: Preparation of 3,3 ‘, 4,4′-benzophenonetetracarboxylic dianhydride by the oxidation of 3,3′, 4,4’-tetramethyl benzophenone |
The first oxidation method consists in treatment with nitric acid of tetra substituted benzophenone [4-6]. The yields of 70-75% of theoretical value (for acid) are achievedby this method.
The second method consists in a liquid-phase catalytic oxidation of tetraalkylbenzophenone with air in the acetic acidusing a metal halide catalysts (Co-Br, Co-Mn-Br, Co-Mn-Mo-Br) in the medium, providing the activation of the catalyst (protonic acids and halogen- substituted aliphatic acids) [7,8]. The yields of 79-96% of theoretical value (for acid) are achieved by second method.
The most economically feasible and available raw materials used to obtain 3,3′,4,4′-tetraalkyl benzophenone are in particular o-xylene, formaldehyde and acetaldehyde. H2SO4or more expensive metal-containing catalysts are usually used as the catalysts(see Figure 5). All sources of these raw materials are widely available.
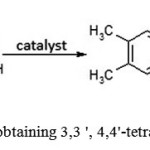 |
Figure 5: Scheme of obtaining 3,3 ‘, 4,4’-tetramethyl benzophenone |
There are data of the use of low temperature methods of synthesis of 3,3′, 4,4′-benzo phenone tetra carboxylic dianhydride [9,10].However, the use of such compounds as sodium hypochlorite [9], and the need for use of a complex synthesis scheme, a large amount of initial reagents and expensive palladium and nickel compounds stilllimits the ability of the widespread use of these synthesis methods.
According to the patent [6] the process of oxidation of 3,3′, 4,4′-tetramethyl benzophenoneis carried out with nitric acid in the temperature range from 150 to 250°C, and the dehydration to obtain benzo phenone tetra carboxylicdian hydride at a temperature of 280 – 320 ° C for 10 hours. Synthesis of the starting aromatic hydrocarbon is carried out by condensation of o-xylene with acetaldehyde in the presence of H2SO4, under relatively mild conditions (T = 10 – 50 ° C, P = 0.6-0.8 MPa), according to the scheme which is similar to that shown in Figure 4. The condensation of o-xylene may be carried out using formaldehyde. Selection of formaldehyde or acetaldehyde is determined by availability, as well as fire and explosion hazard of these reagents.
In laboratory conditions, 3,3′, 4,4′-tetramethylbenzo phenoneis prepared by aluminum tri chloride catalyzed Friedel-Crafts reaction. The method with alkylation of o-xylene with carbon tetrachloride followed by treatment with alkali has been chosen to develop a laboratory technique (see Figure 6) [11].
![Figure 6: Preparation of 3,3 ', 4,4'-tetramethyl-benzophenone by Friedel-Crafts alkylation followed by treatment with alkali [11]](http://www.orientjchem.org/wp-content/uploads/2016/11/Vol32No6_Deve_ANTO_fig6-150x150.jpg) |
Figure 6: Preparation of 3,3 ‘, 4,4’-tetramethyl-benzophenone by Friedel-Crafts alkylation followed by treatment with alkali [11] |
The selected method for the preparation is shown in Figure 7
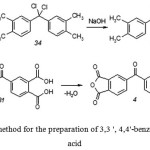 |
Figure 7: The selected method for the preparation of 3,3 ‘, 4,4’-benzophenonetetracarboxylic acid |
Experimental part
Preparation of 3,3′, 4,4′-benzophenonetetra carboxylic acid is carried out in four stages. A series of optimization experiments were conducted for each of successive reactions.
Materials
Carbon tetrachloride, aluminum chloride, sodium hydroxide, potassium permanganate were purchasedfrom Vekton. Magnesium sulfate was also purchasedfrom Vekton and calcined before use. Toluene, o-xylene, pyridine, heptane, diphenyl oxide were purchased from Component-Reaktiv.
Analytical techniques
The resulting products and their purity were identified by NMR spectroscopy, elemental analysis and mass spectroscopy. NMR spectra were recorded on Bruker Avance III HD with an operating frequency of 300 MHz.DMSO-d6 was used as the solvent and tetramethylsilane as internal standard. Elemental analysis was recorded on CHNS-analyzer (EuroVector, Euro EA 3000 model). Mass spectra were obtained using chromatography-spectrometry system “ChromatecKristall 5000.2” with mass-spectrometer detector ISQ Thermo Scientific.
Preparation of 3,3′,4,4′-tetra methyl diphenyl dichloromethane
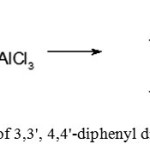 |
Figure 8: Preparation of 3,3′, 4,4′-diphenyl dimethyl dichloromethane Click here to View figure |
A three-necked round bottom flask equipped with magnetic stirrer, a reflux condenser, a thermometer and a dropping funnel was filled with an hydrous aluminum chloride and 22 ml (35 g, 0.23 mol) of carbon tetrachloride, then reaction mixture was cooled with vigorous stirring and a solution of 10 g (0,09 mol) of o-xylene and 6 ml (9.5 g, 0.06 mol) of carbon tetra chloride was added drop wise in such a way that the temperature of reaction mixture did not rise above a certain value. Upon complete addition 20 ml of distilled waterwas added to the reaction mixture through the dropping funnel, the organic layer was separated, washed with distilled water (2 × 20 ml) and dried with anhydrous magnesium sulfate. Then, carbon tetrachloride was distilled under vacuum and the product was obtained as colorless oil, which was used in the next stage without further purification. The results are shown in Table 1.
1H NMR spectrum (DMSO – d6) (δ, ppm): 7.07-7.25 (m, 6H, HAr), 2.43 (s, 12H, CH3)
Table 1: The effect of the temperature conditions and the ratio of raw materialsto yield of 3,3′,4,4′-tetramethyl diphenyl dichloromethane
| № | Temperature mode of adding o-xylene solution (initial and final temperature), ° C | Aluminum chloride | The molar ratio of the reactantso-xylene: aluminum chloride | Yield, % | |
| Weight, g | Quantity, mol | ||||
| 1 | -5 – 0 | 19 | 0,135 | 1:1,5 | 60,0 |
| 12 | 0,09 | 1:1 | 62,0 | ||
| 8 | 0,06 | 1:0,67 | 85,0 | ||
| 6 | 0,045 | 1:0,5 | 57,0 | ||
| 2 | 0 – 5 | 19 | 0,135 | 1:1,5 | 54,0 |
| 12 | 0,09 | 1:1 | 75,0 | ||
| 8 | 0,06 | 1:0,67 | 90,0 | ||
| 6 | 0,045 | 1:0,5 | 64,0 | ||
| 3 | 5 – 10 | 19 | 0,135 | 1:1,5 | 47,0 |
| 12 | 0,09 | 1:1 | 49,0 | ||
| 8 | 0,06 | 1:0,67 | 51,0 | ||
| 6 | 0,045 | 1:0,5 | 42,0 | ||
| 4 | 10 – 15 | 19 | 0,135 | 1:1,5 | 30,0 |
| 12 | 0,09 | 1:1 | 37,0 | ||
| 8 | 0,06 | 1:0,67 | 45,0 | ||
| 6 | 0,045 | 1:0,5 | 34,0 | ||
Table 1 shows that the preparation of 3,3′, 4,4′- tetramethyl phenyldi chloromethane by alkylation of carbon tetra chloride in the presence of an hydrous aluminum chloride should be conducted at a molar ratio of o-xylene: aluminum chloride 1: 0.67 at 0 – 5 ° C.
Preparation of 3,3′,4,4′-tetramethyl benzophenone
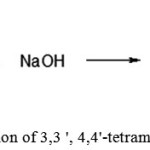 |
Figure 9: Preparation of 3,3 ‘, 4,4’-tetramethyl benzophenone |
A round bottom flask, equipped with magnetic stirrer and reflux condenser was filled with 200 ml of sodium hydroxide solution and 20 g (0.07 mol) of 3,3′,4,4′-tetra methyldiphenyldichloromethane. The reaction mixture was heated while stirring. The reaction mixture was cooled to room temperature and extracted from the aqueous phase with toluene (3 × 50ml), the extracts were combined, washed with distilled water (3 × 50 ml) and dried with anhydrous magnesium sulfate. The solvent was distilled off under vacuum to obtain the product as a white powder, which was used in the next step without further purification. The test results are shown in Table 2.
Elemental analysis. Calculated,%: C 85.67; H 7,61. Found,%: C 85,72; H 7,54.
1H NMR spectrum (DMSO – d6) (δ, ppm): 7.29-7.51 (m, 6H, HAr), 2.38 (s, 12H, CH3).
Table 2: The influence of the concentration of hydrolyzing agent, and reaction time on the yield of 3,3 ‘, 4,4’-tetramethyl benzophenone
| № | Dwell time, hour | The concentration of sodium hydroxide solution,% | Yield, % |
| 1 | 0,5 | 2 | 40,0 |
| 5 | 63,0 | ||
| 10 | 75,0 | ||
| 2 | 1 | 2 | 65,0 |
| 5 | 90,0 | ||
| 10 | 93,0 | ||
| 3 | 2 | 2 | 79,0 |
| 5 | 87,0 | ||
| 10 | 93,0 | ||
| 4 | 3 | 2 | 81,0 |
| 5 | 87,0 | ||
| 10 | 93,0 |
Table 2 shows that the hydrolysis of 3,3′,4,4′-tetramethyldiphenyl dichloromethanehas been carried out with 10% sodium hydroxide solution for 1 hour.
Preparation of 3,3′,4,4′-benzophenone tetracarboxylic acid
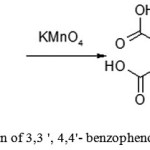 |
Figure 10: Preparation of 3,3 ‘, 4,4’ benzo phenone tetra carboxylic acid |
A four-necked round bottom flask equipped with mechanical stirrer, a reflux condenser, thermometer and funnel for loading bulk solids was filled with 10.7 g (0.045 mol) of 3,3′,4,4′-tetramethylbenzophenone, 64 ml of solvent and 320 ml of water. Then started stirring and heating, the reaction mixture was adjusted to 85 ° C. At this temperature, potassium permanganate (5 – 7 g) was added through the funnel portion wise. Before adding a further portion of potassium permanganate, a sample (2 ml) should be taken from the reactor via a Pasteur pipette, and visually observed the moment by color change of the mixture from violet to muddy brown, when all added potassium permanganate reacted, and only then the following portion should be added. Oxidation is considered to be finished, if a new portion of potassium permanganate introduced was not oxidized for 1 hour. During the experiment, it was found that 60 g (0.38 mol) of potassium permanganate was necessary to fully oxidize the 3,3 ‘, 4,4’-tetramethyl benzophenone.
To convert the excess of potassium permanganate to manganese oxide (IV) 20 ml of ethanol was added to the reaction mixture and stirred at room temperature until disappearance of the purple color. Manganese oxide (IV) formed during the reaction was filtered off and washed with hot water (6 × 50 ml). Basic filtrate and washingwater were combined and water and solvent were evaporated on a rotary evaporator so that the 1/3 of the initial volume of the solution was remained. The resultant filtrate was poured into a glass, cooled to 10 ° C and under stirring concentrated hydrochloric acid was added until acidic reaction on universal indicator. The acidic solution was left overnight at room temperature. The crystals precipitated was filtered and recrystallized from 10% of hydrochloric acid solution. A pyridine solvent should be used for preparation of 3,3 ‘, 4,4’-benzophenonetetracarboxylic acid.
Elemental analysis. Calculated,%: C 56.99; H 2,81. Found,%: C 57,04; H 2,76.
1H NMR spectrum (DMSO – d6) (δ, ppm): 11.31 (s, 4H, COOH), 8.59 (d, 2H, HAr, J = 7.69 Hz), 8.29 (d, 2H, HAr, J = 7.53 Hz), 8.07 (c, 2H, HAr).
Mass spectrum m / z (Irel,%): 322 [M – H4O2] (32,7), 278 [M – CH4O4]
Preparation of 3,3′,4,4′-benzophenone tetracarboxylic dianhydride (Figure 11)
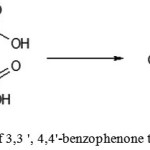 |
Figure 11: Preparation of 3,3 ‘, 4,4’-benzophenone tetracarboxylic dianhydride |
A two-necked round bottom flask equipped with magnetic stirrer, thermometer and Dean-Stark trap with reflux condenser was filled with 5.8 g (0.016 mol) of 3,3′,4,4′-benzophenonetetracarboxylic acid, 15ml ofsolvent (forming an azeotrope with water, separating from the water and not reacting with either an acid or anhydride) was added. Reaction mixture was heatedwhile stirring to boiling, and water was removed from the reaction mixture as an azeotrope mixture. The process was continued until the formation ofclear, light brown solution in the reaction flask and the separation of ~ 0.6 ml of water. The solution in the reactor was cooled to 50 ° C and 24 ml of 1,4-dioxane was added. It was cooled to room temperature. The precipitated crystals were filtered, washed with 10 ml of boiling 1,4-dioxane and dried in vacuo.
According to the results it has beenfound that anhydridization of 3,3′,4,4′-benzophenonetetra carboxylic acid requires high temperatures and therefore high boiling solvent. The reaction should be conducted in the diphenyl etherat a temperature of 230 ° C for 2 hours for the best results.
Elemental analysis. Calculated,%: C 63.37; H 1.88. Found,%: C 63,26; H 1,85.
1H NMR spectrum (DMSO – d6) (δ, ppm): 8.96 (2H, dd), 8.35 (2H, d), 8.19 (2H, d).
Results and Discussion
As a result of studies it has been found an optimum ratio of o-xylene and aluminum chloride in the preparation of 3,3′,4,4′- tetra methyl diphenyl di chloro methane by alkylation with carbon tetra chloride is 1: 0.67 of o-xylene: aluminum chloride at 0-5 ° C. The yield of 3,3′,4,4′-tetra methyl diphenyl di chloro methane under these conditions attains the value 90%.
Optimal conditions for obtaining 3,3′,4,4′-tetramethylbenzophenonewith a yield of 93% were determined, the reaction time was 1 hour, the concentration of sodium hydroxide solution was 10%.
From the series of experiments for obtaining 3,3′,4,4′-benzophenonetetracarboxylic acid by oxidation of 3,3′,4,4′-tetramethylbenzophenonewith potassium permanganate follows that to carry out this process with a maximum yield a molar ratio of 3,3′,4,4′-tetramethylbenzophenoneto potassium permanganate should be 1: 8.4. The maximum yield obtained has been achieved at this ratio and reached 70%.
Preparation of 3,3 ‘, 4,4’-benzophenonetetracarboxylic acid by anhydridization should be performed using diphenyl ether as a solvent. Maximum yield of dianhydride reaches 70%.
All the products obtained at the intermediate and final stages of the developed method have the purity of not less than 90%.
Conclusion
A laboratory procedure for the preparation of intermediate products and 3,3′,4,4′-benzophenontetracarboxylic dianhydride has been developed and optimized. The conditions which allow to achieve yields of more than 70 and up to 95% were chosen at each stage. The available and the least expensive reagents were used in method obtained. The resulting target dianhydride can be used to obtain thermally stable polyimides.
Acknowledgements
Applied researches were carried out with state financial support represented by the Ministry of Education of Russia under the Agreement on granting subsidies No. 14.625.21.0035 of October 27, 2015. (Unique identifier of Applied Scientific Researches (project) RFMEFI62515X0035)
References
- Bessonov, M.I.; Koton, M.M.; Kudryavtsev, V.V.; Laius, L.A; Polyimides: Thermally Stable Polymers. 2nd edition. Plenum. New York.1987.
CrossRef - Dine-Hart, R.A.; Wright, W.W. J. Appl. Polym. Sci. 1967,. 11.. 5, 609-627.
CrossRef - Bacosca, I.; Hamciuc, E.; Bruma, M.; Ronova, I.A. J. Iran. Chem. Soc.2012, 9.. 6, 901-910.
CrossRef - McCracken, J.H.; Schulz, J.G.D. Patent US3078279,1960.
- McCracken, J.H.; Schulz, J.G.D. Patent US3297727,1963.
- Onopchenko, A.; Schulz, J.G.D. Patent US4173573,1978.
- Aleksandrov, V.N.; Gitis, C. C .; Pugacheva, S. A .; Golubev G.S. Patent SU 300457, 1969.
- Mavlyutova, N.K..; Khannanov, T.M. .; SharipovA.Kh.Patent SU232237, 1967.
- Maruyama, Y.; Negishi, J.; Murata, K.; Katsuhara, Y. Patent US4599455, 1983.
- Chuang, C.-H. Patent US7381849, 2004
- Mironov, G.S.; Chernyakhovskaya N.A. .; Farberov M.I. .; Tyuleneva, I.M.; Rusakova, M. S. Zhurnalprikladnoikhimii. 1970.. 43.. 3, 620-627.

This work is licensed under a Creative Commons Attribution 4.0 International License.









