Adsorption Study of Pb(II), Cd(II), Hg(II) And Cr(III) Onto Calix[4]Resorcinarene Derivative
Chairil Anwar1*, Jumina1, Dwi Siswanta1, Sri Juari Santosa1, Riani Kusuma Dewi1 and Muhamad Idham Darusalam Mardjan2
1Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Gadjah Mada, Sekip Utara Yogyakarta 55281.
2Department of Chemistry and Applied Chemistry, Saga University, 1-Honjo, Saga 840-8502, Japan.
Corresponding Author E-mail: chanwar@ugm.ac.id
DOI : http://dx.doi.org/10.13005/ojc/320606
In this study, the removal of several heavy metal ions of Pb(II), Cd(II), Hg(II) and Cr(III) from aqueous medium via sorption process onto calix[4]resorcinarene derivative was investigated. The used adsorbent was highly oxygenated calix[4]resorcinarene namely C-4-hydroxyphenylcalix[4]resorcinarene. Several adsorption parameters were studied including pH, adsorbent dosage, interaction time as well as the kinetic studies. While the maximum removals of Pb(II), Cd(II) and Hg(II) were observed in pH 5, the removal of Cr(III) reached the maximum value at pH 6. The optimum adsorbent dosages for Pb(II), Hg(II) and Cr(III) were 0.025 g, whereas that for Cd(II) was 0.05 g. The kinetic data were evaluated by using three kinetic models of first order model of Santosa, pseudo-first order of Lagergren and pseudo-second order of Ho. The results showed that the adsorption of these metal ions could be well described with Ho's pseudo-first order model.
KEYWORDS:Calix[4]resorcinarene derivative; adsorption; heavy metal ions
Download this article as:| Copy the following to cite this article: Anwar C, Jumina J, Siswanta D, Santosa S. J, Dewi R. K, Mardjan M. I. D. Adsorption Study of Pb(II), Cd(II), Hg(II) And Cr(III) Onto Calix[4] Resorcinarene Derivative. Orient J Chem 2016;32(6). |
| Copy the following to cite this URL: Anwar C, Jumina J, Siswanta D, Santosa S. J, Dewi R. K, Mardjan M. I. D. Adsorption Study of Pb(II), Cd(II), Hg(II) And Cr(III) Onto Calix[4]Resorcinarene Derivative. Orient J Chem 2016;32(6). Available from: http://www.orientjchem.org/?p=25448 |
Introduction
A great concern has been still given to the heavy metal contamination in aquatic environment. Heavy metals were notoriously known due to their toxicity and bioaccumulation properties. In addition, several health damage were correlated to the heavy metal contamination.1–4Therefore, an effective treatment to remove the heavy metal ions from water or wastewater is highly required.
Several methods have been studied for the removal of heavy metals from aqueous medium such as adsorption, ion exchange, membrane filtration, chemical precipitation, electrochemical treatment as well as photocatalysis.5,6 However, some of these techniques suffered from several drawbacks such as high operational cost and time as well as energy consumption.5
Adsorption is considered to be a simple, effective and cheap method for heavy metal removal particularly at water medium with low metal ions concentrations and wastewater.Beside its high metal binding capacity, the adsorbent can be regenerated by suitable desorption process.7Various adsorbent has been reported in the removal of heavy metal ions. For instance, activated carbon,8 zeolite,9 polymer,10 biosorbent,11 and macrocycle.12–15
Calixarene is one class of macromolecules which has been widely applied in the field of host-guest chemistry due to the existence of molecular cavity and the chelating atoms.16 Calixarene also offers flexibility in structural design, such as changing the cavity size and incorporating various functional group which allowed the formation of variety of macromolecules with different physical and chemical properties.
Calixarene derivative namely calix[4]resorcinarene is a cyclic olygomer of resorcinol linked by methylene bridges. The application of different kind of calix[4]resorcinarene derivatives in the removal of heavy metals has been demonstrated in the literature.17–19 In this report, We conducted the adsorption study of some selected heavy metal cations of Pb(II), Cd(II), Hg(II) and Cr(III) onto C-4-hydroxyphenylcalix[4]resorcinarene adsorbent.
Experimental Part
Material and Instrumentation
C-4-Hydroxyphenylcalix[4]resorcinarene adsorbent was synthesized based on previous study(Figure 1).The metal salts of Pb(NO3)2, Cd(NO3)2, Hg(NO3)2 and Cr(NO3)3.9H2O were purchased from E. Merck. The metal solutions with certain concentration were prepared by solubilizing the nitrate salts with deionized water. Atomic absorption spectrometer (AAS, Perkin Elmer 3110 USA) were used to determine the metal concentration in the adsorption study. The pH value was measured by pHmeter (200A orion), where the pH of the solution was adjusted by the solutions of HCl (0.1 M) and NaOH (0.1 M).
Adsorption Study
The adsorption study was conducted in batch system. In general, 10 mL of metal solution (10 mg/L) was placed in the Erlenmeyer. Having adjusted the pH value, the adsorbent (0.1 g, 100 mesh) was added into the solution and the mixture was shaken at the ambient temperature for certain period of time. In addition, the blank experiments were also carried out. The mixture was filtered and the filtrate was analyzed by using AAS to determine the metal concentration after the adsorption process.
The Eq. 1 was utilized to calculate the adsorption capacity for each experiment.
![]()
where Co and Cf were the initial concentration of metal ion and the final concentration of metal ion after certain period of time, respectively. V was the volume of metal solution in liter and W was the mass of the adsorbent in gram. There were three adsorption parameters which were evaluated including pH, adsorbent dosage and interaction time.
In the kinetic study, first-order of Santosa, pseudo second order of Lagergren and pseude-second order of Ho were tested against the experimental data to determine the kinetics of the adsorption process. While the Santosa model (Eq. 2) was based on the concentration of adsorbate in the aqueous phase,20 both Lagergren (Eq. 3) and Ho (Eq. 4) were based on the concentration of the adsorbate in the solid phase.21,22

![]()

where t was the adsorption time (minute) and K was the equilibrium constant (mol/L). The values of qe and qt were the amounts of adsorbed metal (mg/g) at equilibrium and contact time t; k (minute-1), k1 (mg.g-1.min-1) and k2 (g.mg-1.min-1) were the rate constants for first, pseudo-first and pseudo-second orders, respectively.
Results and Discussion
The design of calix[4]resorcinarene as a good adsorbent may be achieved by incorporating chelating atoms to the skeleton. Various functional groups such as hydroxy, thiol, amine and carboxylic acid derivatives has been used as binding sites to the metal ions.23–30In this report, the highly oxygenated calix[4]resorcinarene derivative namely C-4-hydroxyphenylcalix[4]resorcinarene(Figure 1) was employed in the removal of Pb(II), Cd(II), Hg(II) and Cr(III) ions. The adsorption study was performed by investigating several parameters such as pH, adsorption dose and interaction time as well as the kinetic study.
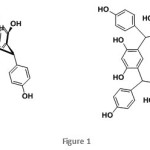 |
Figure 1 Click here to View figure |
Effect of pH
pH is one of important parameters in the adsorption process since the adsorbent’s surface charge and metal speciation were dependant on pH value.31 To study the effect of pH on the adsorption of heavy metals on calix[4]resorcinarene derivative, We conducted the experiments in the pH range 2-6(Figure 2). The results showed that the optimum pH for Cr(III) was 6 and that fornPb(II), Cd(II) and Hg(II) were 5.
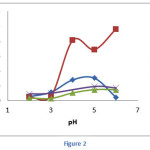 |
Figure 2 |
As displayed in Figure 2, the adsorption of the metal ions had similar trend. The low adsorption capacities were observed at low pH and the capacities increased by increasing the pH. These results may be rationalized by considering the both adsorbent surface charge and metal speciation.
Hydroxyphenylcalix[4]resorcinarene mostly contained phenolic groups as the binding site. Phenol is a weak acid (pKa = 9.95 in water) which will be mainly existed as its protonated form (R-OH2+) at low pH. Increasing the pH may decrease the degree of protonation and led to the formation of free phenol (R-OH).
Concerning the speciation of metal at different pH, both Pb(II) and Cd(II) are in the form of Pb2+ and Cd2+ at pH lower than 6.32 While the major species of Cr(III) at low pH is Cr3+, Cr3(OH)45+ and CrOH2+ are observed in the pH range 4-6.33 The predominant Hg species at pH < 4 is HgCl2 and at pH > 4 is Hg(OH)2.34
At low pH, both adsorbent and metal ions were existed as positively-charged species. Therefore, the electrostatic repulsion might occur and reduce the adsorption capacity. In addition, the proton may significantly compete with metal ions to bind the active sites of adsorbent. At higher pH value, the adsorption capacities tended to increase due to relatively high amount of free phenol which may coordinate with the metal ions. Moreover, the slight increase of adsorption capacity of Hg(II) at higher pH was presumably due to the possibility of hydrogen bond formation between phenolic groups on adsorbent surface and Hg(OH)2.
Effect of Adsorption Dose
The effect of adsorption dose on the adsorption of heavy metal ions on calix[4]resorcinarene derivative was carried out by varying the mass of adsorbent in the range 0.025-0.150 g (Figure 3). Similar trends were observed for all tested metal ions. The adsorption capacity tended to decrease with the increase of adsorbent dose. At higher dose of adsorbent, the aggregation of the adsorbent may occur and reduce the number of binding site as well as the adsorption capacities. The optimum dose for the adsorption of Cr(III), Hg(II) and Pb(II) were 0.025 g, whereas that for Cd(II) was 0.050 g.
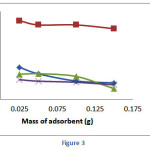 |
Figure 3 Click here to View figure |
Effect of Interaction Time
Figure 4 depicted the adsorption profile of Pb(II), Cd(II), Hg(II) and Cr(III) onto calix[4]resorcinarene derivative as the function of contact time. It was observed that hte adsorption capacities significantly increased at the first 5 minutes and gradually increase until the equilibrium were obtained. The rapid increase of capacities was due to the high vacant binding sites. The equilibrium time for Pb(II), Cd(II), Hg(II) and Cr(III) were 30, 10, 30, 15 minute, respectively. Having reached the equilibrium, the capacities tend to decreasedue to the saturation of the binding sites of the adsorbent.
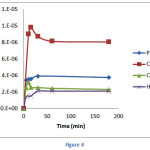 |
Figure 4 Click here to View figure |
Kinetic study
In order to study the adsorption mechanism, three kinetic models were applied. They were first order of Santosa, pseudo-first order of Lagergren and pseudo-second order of Ho. The kinetic parameters were presented in Table 1.
The results showed that the highest linearity (R2) for all metal ions was obtained from the pseudo-second order. Moreover, the qe values calculated from Ho’s model were close to the experimental qe values indicating that the adsorption of the heavy metal ions could be better described by this kinetic model.
Table 1
|
Kinetic Parameters |
Metal |
|
|||
|
Pb(II) |
Cd(II) |
Hg(II) |
Cr(III) |
||
|
qe (mg/g) |
0.811 |
0.285 |
0.420 |
0.578 |
|
| Santosa-Muzakky | k (1/min) |
0.006 |
0.002 |
0.012 |
0.005 |
| r2 |
0.823 |
0.648 |
0.886 |
0.858 |
|
| Pseudo first order | k1 (1/min) |
0.0082 |
0.0278 |
0.0013 |
0.0298 |
| qe (mg/g) |
0.1294 |
0.0147 |
0.1391 |
0.3252 |
|
|
Pseudo second order |
k2 (g/mg.min) |
2.0408 |
1.8289 |
1.7509 |
3.7733 |
| qe (mg/g) |
0.7784 |
0.2552 |
0.3838 |
0.5813 |
|
| r2 |
0.9999 |
0.9996 |
0.9991 |
0.9999 |
|
| h |
1.2365 |
0.1191 |
0.2579 |
1.2786 |
|
The adsorption kinetic rate k2 could be obtained from the plot of t/qt vs t and followed the order Cr(III) > Pb(II) > Cd(II) > Hg(II). The order might be explained by using Pearson Hard Soft Acid Base principle.35 The phenol groups on the calix[4]resorcinarene was classified as hard base. Due to the high charge density, Cr(III) was classified as hard acid. As the consequence, the interaction between Cr(III) and binding sites of adsorbent was favorable. According to HSAB theory, Pb(II) was moderate acid while Cd(II) and Hg(II) were soft acid. Therefore, the rate constants k2 were lower than that of Cr(III).
Conclusion
A highly oxygenated C-4-Hydroxyphenylcalix[4]resorcinarene has been employed in the adsorption of Pb(II), Cd(II), Hg(II) and Cr(III). It was found that the optimum pH values for Pb(II), Cd(II) and Hg(II) were 5 and the optimum pH for Cr(III) was 6. The optimum removal of Pb(II), Hg(II) and Cr(III) were obtained when 0.025 g of sorbent was used and the removal of Cd(II) reached the maximum value at the dose of 0.05 g. The kinetic studies showed that adsorption of all metal ions on calix[4]resorcinarene derivatives followed pseudo-second order of Ho.
References
- Maharia, R. S.; Dutta, R. K.; Acharya, R.; Reddy, A. V. R. J. Environ. Sci. Health Part B2010, 45 (2), 174–181.
CrossRef - Ahmad, R.; Kumar, R.; Laskar, M. A. Environ. Sci. Pollut. Res.2013, 20 (1), 219–226.
CrossRef - El-Moselhy, K. M.; Othman, A. I.; Abd El-Azem, H.; El-Metwally, M. E. A. Egypt. J. Basic Appl. Sci.2014, 1 (2), 97–105.
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B. B.; Beeregowda, K. N. Interdiscip. Toxicol.2014, 7 (2).
CrossRef - Barakat, M. A. Arab. J. Chem.2011, 4 (4), 361–377.
CrossRef - Fu, F.; Wang, Q. J. Environ. Manage.2011, 92 (3), 407–418.
CrossRef - Lata, S.; Singh, P. K.; Samadder, S. R. Int. J. Environ. Sci. Technol.2015, 12 (4), 1461–1478.
CrossRef - Kang, K. C.; Kim, S. S.; Choi, J. W.; Kwon, S. H. J. Ind. Eng. Chem.2008, 14 (1), 131–135.
CrossRef - Basaldella, E. I.; Vázquez, P. G.; Iucolano, F.; Caputo, D. J. Colloid Interface Sci.2007, 313 (2), 574–578.
CrossRef - Wang, L.-Y.; Wang, M.-J. ACS Sustain. Chem. Eng.2016, 4 (5), 2830–2837.
CrossRef - Sud, D.; Mahajan, G.; Kaur, M. Bioresour. Technol.2008, 99 (14), 6017–6027.
CrossRef - Li, H.-B.; Chen, Y.-Y.; Liu, S.-L. J. Appl. Polym. Sci.2003, 89 (4), 1139–1144.
CrossRef - Tabakci, M.; Erdemir, S.; Yilmaz, M. J. Hazard. Mater.2007, 148 (1–2), 428–435.
CrossRef - Tabakci, M.; Yilmaz, M. J. Hazard. Mater.2008, 151 (2–3), 331–338.
CrossRef - Nakajima, L.; Yusof, N. N. M.; Kobayashi, T. Arab. J. Sci. Eng.2015, 40 (10), 2881–2888.
CrossRef - Gutsche, C. D. Calixarenes revisited; Monographs in supramolecular chemistry; Royal Soc. of Chemistry: Cambridge, 1998.
- Jumina; Sarjono, R. E.; Paramitha, B.; Hendaryani, I.; Siswanta, D.; Santosa, S. J.; Anwar, C.; Sastrohamidjojo, H.; Ohto, K.; Oshima, T. J. Chin. Chem. Soc.2007, 54 (5), 1167–1178.
- Jumina, J.; Sarjono, R. E.; Siswanta, D.; Santosa, S. J.; Ohto, K. J. Korean Chem. Soc.2011, 55 (3), 454–462.
CrossRef - Kesuma, E.; Jumina, J.; Ohto, K.; Siswanta, D. Orient. J. Chem.2016, 32 (2), 769–775.
CrossRef - Santosa, S. J.; Siswanta, D.; Sudiono, S.; Sehol, M. Surf. Sci.2007, 601 (22), 5148–5154.
CrossRef - Ho, Y. .; McKay, G. Process Biochem.1999, 34 (5), 451–465.
CrossRef - Ho, Y. J. Hazard. Mater.2006, 136 (3), 681–689.
CrossRef - Adhikari, B. B.; Kanemitsu, M.; Kawakita, H.; Jumina; Ohto, K. Chem. Eng. J.2011, 172 (1), 341–353.
CrossRef - Ahmad, R.; Kumar, R.; Laskar, M. A. Environ. Sci. Pollut. Res.2013, 20 (1), 219–226.
CrossRef - Handayani, D. S.; Jumina; Siswanta, D.; Mustofa; Ohto, K.; Kawakita, H. Indones. J. Chem2011, 11 (2), 191–195.
- Adhikari, B. B.; Gurung, M.; Chetry, A. B.; Kawakita, H.; Ohto, K. RSC Adv.2013, 3 (48), 25950.
CrossRef - Adhikari, B. B.; Ohto, K.; Schramm, M. P. Chem. Commun.2014, 50 (15), 1903.
CrossRef - Mulya, P. W.; Jumina; Siswanta, D.; Gusti Ngurah, B. I. M. Adv. Mater. Res.2014, 1043, 129–132.
- Al-Trawneh, S. Jordan J. Earth Env. Sci.2015, 7 (1), 1–9.
- Siswanta, D.; Jumina; Anggraini, M.; Mardjan, M. I. D.; Mulyono, P.; Ohto, K. Int. J. App. Chem.2016, 12 (1), 11–22.
- Pagnanelli, F.; Esposito, A.; Toro, L.; Vegliò, F. Water Res.2003, 37 (3), 627–633.
CrossRef - Berber-Mendoza, M. S.; Leyva-Ramos, R.; Alonso-Davila, P.; Mendoza-Barron, J.; Diaz-Flores, P. E. J. Chem. Technol. Biotechnol.2006, 81 (6), 966–973.
CrossRef - Santos, V. C. G. D.; Salvado, A. de P. A.; Dragunski, D. C.; Peraro, D. N. C.; Tarley, C. R. T.; Caetano, J. Quím. Nova2012, 35 (8), 1606–1611.
CrossRef - Yardim, M. F.; Budinova, T.; Ekinci, E.; Petrov, N.; Razvigorova, M.; Minkova, V. Chemosphere2003, 52 (5), 835–841.
CrossRef - Pearson, R. G. J. Chem. Educ.1968, 45 (9), 581.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









