Studies on Alumina Incorporated Polyesteramide Derived from Meliaazedarach Seed Oil
A. Hasnat1*, M. Naseem1,2 and S. A. Ahmad1
1Natural products and Polymer Research Laboratory, G. F. College (Affiliated to M.J.P. Rohilkhand University) Shahjahanpur- 242001,U.P., India.
2Applied Chemistry Laboratory, University Polytechnic, Integral University, CampusShahjahanpur- 242001, U.P., India.
*Corresponding Author E-mail: hasnatgfc@rediffmail.com
DOI : http://dx.doi.org/10.13005/ojc/320554
Polyesteramide resin (MAPEAM) was prepared from N,N-bis(2-hydroxy ethyl) Meliaazedarach oil fatty amide (HEMAFA) a precursor of natural renewable resource using polycondensation reaction with maleic acid. With the view to improve the physico-mechenical properties aluminium was incorporated in backbone of the polymer to obtain the alumina incorporated polyesteramide resin of Meliaazedarach seed oil (Al-MAPEAM). The physico-chemical analyses and spectroscopic techniques were used for the characterization of Al-MAPEAM polymeric resin. The film properties of the Al-MAPEAM were also investigated in different corrosive environments as per standard reported methods. Studies shows that syntheses ofaluminium incorporated polyesteramide using Meliaazedarach seed oil as a starting material provides a more practicable utilization to it.
KEYWORDS:Meliaazedarach seed oil; Alumina-filled polyesteramides; coating materials; vegetable oil
Download this article as:| Copy the following to cite this article: Hasnat A, Naseem M, Ahmad S. A. Studies on Alumina Incorporated Polyesteramide Derived from Meliaazedarach Seed Oil. Orient J Chem 2016;32(5). |
| Copy the following to cite this URL: Hasnat A, Naseem M, Ahmad S. A. Studies on Alumina Incorporated Polyesteramide Derived from Meliaazedarach Seed Oil. Orient J Chem 2016;32(5). Available from: http://www.orientjchem.org/?p=22004 |
Introduction
Polyesteramide resins are amide modified alkyds embedded with both amide and ester groups, reported for improved performances in terms of hardness, ease of drying, water vapour resistance over normal alkyds which contain only ester linkages1-3.These resins are largely used as a coating materials or binder for paints used to protect the metals from environmental corrosion and wood from biological organism4,5.Furthermore, incorporation of metal in the polymers appreciably enhances the physico-mechanical properties in terms of hardness, resistance to scratch, flexibility and bending5 as well as also reduces the curing temperature6.
The petrochemicals have been used as primary raw material for the synthesis of commercially important polymers for the last few decades7-9.In recent years search for alternative feed stock has been stimulated due to the depletion of petroleum reserves on one hand and the increasing demand for petroleum products on the other10,11.The vegetable oils especially those obtained from different seeds extensively utilized in developing polymeric resins like alkyds, polyesteramides, epoxies and many others1,5. These resins have got prominent applications in field paint and coatings industries3. To reduce the consumption of traditional vegetable oils like sunflower, linseed, coconut, soybean, it is required to utilize the non-traditional and non-edible vegetable oil in the polymer syntheses.
Meliaazedarach (Bakain) is one of the oil seed bearing plants and largely grown in almost all climatic conditions in the country12. High iodine value of the triglyceride oil encourages us to utilize in the synthesis of polymeric materials of film forming ability. Survey of literature shows that Meliaazedarach seed oil is abundantly available in the country still waits for practicable utilization especially in the polymer synthesis12-14. Keeping these facts in mind in present work efforts have been made to utilize the Meliaazedarach seed oil in the synthesis of alumina incorporated polyesteramideusing maleic acid as a dibasic acid (Al-MAPEAM) with the objective to utilization of renewable resource in making profitable materials. The synthesized polymeric resin was characterized by physic-chemical and spectral analyses. The film properties of the Al-MAPEAM were also investigated in different corrosive environment as per standard reported methods.
Experimental
Materials
Oil was extracted from air dried and crushed seeds of Meliaazedarach(collected from the R.T.O. office compound, Shahjahanpur, India) through a soxhlet apparatus, using petroleum ether (boiling point range 60-80 oC) as a solvent. The results of physic-chemical characterization and fatty acid composition Meliaazedarch seed oil9,12 are summarized in Table 1. Maleic acid, aluminium hydroxide, xylene, methanol and diethanolamine were used of analytical grade (S.D. Fine Chemicals, India).
Table 1: Characterization of MASO, HEMAFA, MAPEAM and Al-MAPEAM
| S.No. | Characterization | MASO | HEMAFA | MAPEAM | Al-MAPEAM |
| 1. | Oil content | 40 % | – | – | – |
| 2. | Gardener colour | 6 | 6 | 8 | 7 |
| 3. | Specific gravity | 0.930 | 0.938 | 0.940 | 0.950 |
| 4. | Refractive index | 1.4691 | 1.4697 | 1.5080 | 1.5090 |
| 5. | Iodine value | 134.7 | 65.8 | 37.8 | 36.8 |
| 6. | Acid value | 4.45 | – | 4.60 | 1.8 |
| 7. | Saponification value | 190.8 | – | 152 | 146 |
| 8. | Fatty acid Composition | ||||
| Saturated(Palmitic and Stearic) | 11.4% | ||||
| Unsaturated(oleic and linoleic) | 88.6% |
Syntheses
N, N-bis (2-hydroxyethyl) Meliaazedarach oil fatty amide (HEMAFA)
HEMAFA was prepared as per reported method for the aminolysis of vegetable oils13. Diethanolamine (0.32 mole) and sodium methoxide (0.007 mole) were taken in a four necked round bottom flask fitted with an electrical stirrer, a thermometer, a dropping funnel and a condenser. The reaction mixturewas heated upto 120 oC. The Meliaazedarachseed oil(0.1 mole) was added drop wise into the reaction mixture over a period of 60 minutes. The reaction was further continued for an hour under the same condition. The progress of reaction was monitored by thin layer chromatography (TLC).After the completion of reaction the end product was allowed cooled down at room temperature under continuous string. The product was dissolved in diethyl ether and washed with 5-wt% aqueous NaCl solution and dried over anhydrous sodium sulphate. The ethereal solution was filtered and excess solvent was removed in a rotary vacuum evaporator under reduced pressure to obtain HEMAFA.
Meliaazedarachpolyesteramide from Maleic Acid (MAPEAM)
MAPEAM was synthesized as per poly (condensation) technique between diol and dibasic acid115.HEMAFA and maleic acid in equal molar ratio along with xylene as a solvent were placed in a four necked round bottom flask fitted with a Dean-Stark trap, a thermometer and a mechanical stirrer. Reaction mixture was heated up to 180 oC under continuous stirring and maintain till the completion of reaction. The progress of reaction was monitored by taking acid value at regular intervals17. After the completion of reaction, the reaction product was allowed to cool at ambient temperature under string. The end product was taken out from the reaction flask and excess of xylene was removed in a rotary vacuum evaporator under reduced pressure to obtain MAPEAM.
Synthesis of Alumina Incorporated Polyesteramide(Al-MAPEAM)
Alumina was incorporated in MAPEAM by reacting the resin with Al(OH)3. The mixture of Al(OH)3 (0.006 mole) and MAPEAM (0.05 mole) along with 50 ml xylene was heated in the aforementioned setup at the rate of 10 oC/min upto 60 oC. This temperature was maintained for one hour, followed by an increase in temperature upto 160 ± 5 oC and maintain till the completion of reaction. TLC was used to monitor the progress of reaction. After the completion of reaction, the end product was allowed to cool down at ambient temperature under stirring and diluted in ether, then washed with 5-wt % aqueous NaCl solution. The product was dried over anhydrous sodium sulphate and the excess of solvent was removed in a rotary vacuum evaporator under reduced pressure to obtain the Al-MAPEAM polymeric resin.
Characterization
Physico-chemical characterizations like refractive index, specific gravity, acid value, iodine value of polymeric materials were performed as per standard reported laboratory methods16. Spectral analyses of polymer samples were used for the structural elucidation. FT-IR spectrum of the polymer sample was recorded on FT-IR spectrophotometer (Perkin-Elmer Cetus instruments, Norwalk CT, USA) using a NaCl cell. 1H-NMR and 13C-NMR spectra were recorded on JEOL GSX 300 MHz FX-1000 spectrometer, using tetramethylsilane (TMS) as an internal reference,where asdeuterated chloroform was used as a solvent.
Preparation of Coatings
Coatings of Al-MAPEAM polymeric resin were applied on mild steel coupons, 70x25x1 mm size for physico-mechanical test and 30x10x1 mm size for chemical/corrosion resistance test13. The mild steel strips were polished on various grade of silicon carbide papers, then washed with distilled water, degreased with alcohol and carbon tetrachloride. The 60-wt% solution of Al-MAPEAM resin was applied on these coupons by brush technique13.Coated samples were baked at 200 oC for 10 minutes. Coating thickness were measured by Elcometer and found between 70± 5µm. Physic-mechanical properties of polymeric films like scratch hardness tests (BS 3900), bending tests on conical mandrels and impact resistance tests (IS: 101 part 5/Sec.31988) were investigated. Chemical resistance tests of the coating samples were carried out in water, acid (3-wt % HCl), alkali (2-wt% NaOH) and salt (3.5-wt%) by placing them in 3 in. diameter porcelain dishes, in aforementioned media. The coated samples were examined at usual intervals until coatings showed visual evidence of softening, deterioration in gloss, discoloration16.
Results and Discussion
Figure 1 (a,b,c) depict the reaction schemes for the synthesis of HEMAFA, MAPEAM and Al-MAPEAM. The MAPEAM was synthesized by the aminolysis of MASO with diethanol amine followed by the poly (condensation) polymerization of HEMAFA with maleic acid. MAPEAM was then reacted with Al(OH)3 to obtain Al-MAPEAM polymeric resin. The acid values of the reaction mixture were decreases progressively during the conversion of HEMAFA to MAPEAM and then MAPEAM to Al-MAPEAM. This is due to formation of repeating ester linkages during the condensation polymerization and formation of aluminium carboxylate linkages.
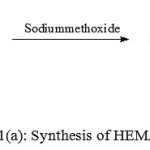 |
Figure 1(a): Synthesis of HEMAFA
|
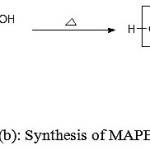 |
Figure 1(b): Synthesis of MAPEAM
|
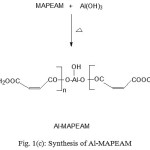 |
Figure 1(c): Synthesis of Al-MAPEAM
|
The FT-IR spectrum of the Al-MAPEAM shows the band of alcoholic group at 3564 cm-1 (broad band of a primary alcohol), CH2 asymmetric and symmetric stretching band at 2920 and 2864 cm-1 respectively17. The bands for carbonyls of repeating ester linkage and amide are observed at 1752 cm-1and 1670 cm-1respectively. The CN stretching band (C-N group) appears at 1470 cm-1, whereas the-C-O-C-asymmetric and symmetric bands are observed at 1380 cm-1 and 1270 cm-1.The 1H-NMR spectrum of Al-MAPEAM shows the peak at δ = 0.84-0.88 ppm for terminal methyl group. A broad peak of chain CH2 group appears at δ = 1.34-1.38 ppm, whereas peaks for the protons attached to double bonded carbons appear at δ=5.38-5.40 ppm, supporting the structure of Al-MAPEAM as shown inFigure 1.13C-NMR spectrum shows the characteristic signal of carbonyl of ester at δ = 182 ppm confirms the formation of ester linkages17. The additional signal such as carbonyl of amide appears at δ = 178 ppm. The peaks for different CH2 groups of the fatty acid chain appear at δ = 32-26 ppm, the peak for double bonded carbon of a fatty acid chain appears at δ = 129.8 and δ = 129.2 ppm. Terminal methyl group of fatty acid chain appears at 16 ppm.
Coating Properties
Coatings of Al-MAPEAM were developed on standard size of mild steel strips for the evaluation of physico-mechanical and chemical/corrosion resistance properties. It has been observed that optimum baking time and temperature for the Al-MAPEAM coating system is 200 oC and 10 minutes. Coatings of Al-MAPEAM were passing the bending test on 1/8 inch conical mandrel; no visual cracks were noticed like other vegetable oil based polymeric coating materials7,9. Coatings of Al-MAPEAM show remarkably high values for scratch hardness and impact resistance, reasonably due to incorporation of aluminium which increase the chain length of polymer, ultimately cohesive forces among the polymeric chain (Table2). The presence of pendent hydroxyl groups on the aluminium in the polymer backbone also responsible for the better adhesion between polymeric chain and surface of metal. The Table 2 indicates that the coatings of Al-MAPEAM show the good protection ability in water, acid solutions and salty environment. These are reasonably due the presence of aluminium in the backbone of the polymer which provides high cross-linking density and better adhesion towards metal surfaces.
Table 2: Coating properties of Al-MAPEAM
| Test | Al-MAPEAM |
|
Physico-mechanical properties |
|
| Bending test (1/8 in) | Passes |
| Gloss at 45o | 105 |
| Impact resistance (lb/in) | 250 |
| Scratch hardness (Kg) | 3.0 |
|
Chemical/corrosion resistance* |
|
| H2O (10 days) | E |
| HCl (3-wt%) 10 days | E |
| NaOH (2-wt%) 2 hrs | C |
| NaCl (3.5-wt%) 10 days | D |
*A= Film detached; B= Film partially detached; C=Loss in gloss; D= Slight loss in gloss; E= Unaffected
Conclusions
The synthesis of Al-MAPEAM from Meliaazedarach seed oils provides a more profitable utilization of non-traditional, non-edible and renewable resource. The synthesize resin was characterized by spectral studies as well as by physico-chemical analyses. The physico-mechanical performances and chemical/corrosion resistance abilities of Al-MAPEAM were also investigated.The study concludes that the synthesis of Al-MAPEAM resin provides a fruitful route for the utilisation of Meliaazedarach seed oil, rot away in every season.
Acknowledgements
The authors are grateful to the Authorities of G.F.College, Shahjahanpur and Honourable Vice-Chancellor, Prof. S.W.Akhtar, of the Integral University, Lucknow for providing research facilities and encouragement.
References
- Zafar, F., Ashraf, S.M., Ahmad, S. Prog. Org. Coat.2004, 51 250.
- Ahmad S, Ashraf S M, Naqvi F, Yadav S and Hasnat A, J. Polym. Mater. 2001, 18, 53.
- Ahmad, S., Ashraf, S.M., Hasnat, A., Yadav, S., Jamal, A. J. Appl.Polym. Sci. 2001,82 1856.
- Ansari, S. H., Imran G., Naseem M., Ahmad, S. A. and Hasnat, A. Orient. J. Chem.2012, 28,607.
- Ansari, S. H., Naseem M., Hasnat, A. and Ahmad, S. A.Biosici. Biotech. Res. Asia 2011, 8, 829.
- Li, F., Henson, M.V. andLarock, R.C. Polymer2001,42, 1567.
- Jayakumar, R., Raj Kumar, M., Nagendran, R. andNanjundan S. J. Appl.Polym. Sci.2002, 85,1194.
- Alam, M., Sharmin, E., Ashraf, S. M. and Ahmad, S. Prog. Org. Coat. 2004, 50, 224.
- Sharmin, E., Imo, L., Ashraf, S. M. and Ahmad, S. Prog. Org. Coat.2004, 50, 47.
- Ahamad, S., Imran, G., Ahmad, S.A. and Hasnat, A. Orient. J. Chem.2015, 31, 1169.
- Abdalla O. M., Ludwick A., Mitchell T. Polymer 2003,44, 7353.
- Lochab, B., Varma, I. K. And Bijwe, J. Adv. Mater. Phys. and Chem.2012, 2, 221.
- Ambasta S. P., The Useful Plants of India, CSIR, India 1994
- Ahmad, S., Ashraf, S.M., Naqvi, F., Yadav, S. and Hasnat A. Prog. Org. Coat.2003, 47 95.
- Islam, M.R., Beg, M.D.H. and Jamari, S.S. J. Appl. Polym Sci.2014, 131, 40787.
- Sharmin, E., Imo. L., Ashraf, S.M. and Ahmad, S. Prog. Org. Coat.2004,50, 47.
- Silverstein R M, Bassler G C &Morril T C, Spectroscopic Identification of Organic Compounds, 5thedn., John Wile y & Sons, New York, 1991.

This work is licensed under a Creative Commons Attribution 4.0 International License.









