Proton-Exchange Membranes Based on Sulfonated Polymers
Yulia Sergeevna Sedesheva, Vitaly Sergeyevich Ivanov, Alyona Igorevna Wozniak and Anton Sergeyevich Yegorov*
Federal State Unitary Enterprise «State Scientific Research Institute of Chemical reagents and High Purity Chemical Substances»(FSUE “IREA”) 107076, Bogorodskyval, 3. Moscow. Russia.
Corresponding Author E-mail: egorov@irea.org.ru
DOI : http://dx.doi.org/10.13005/ojc/320501
Review is dedicated to discussion of different types of proton-exchange membranes used in fuel cells (FC). One of the most promising electrolytes is polymer electrolyte membrane (PEM). In recent years, researchers pay great attention to various non-fluorinated or partially fluorinated hydrocarbon polymers, which may become a real alternative to Nafion. Typical examples are sulfonatedpolyetheretherketones, polyarylene ethers, polysulphones, polyimides. A class of polyimides-based hydrocarbon proton-exchange membranes is separately considered as promising for widespread use in fuel cell, such membranes are of interest for our further experimental development.
KEYWORDS:The fuel cell (FC); a membrane-electrode assembly (MEA); proton-exchange (electrolytic) polymer membrane (PEM); Nafion; polyimides; sulfonated polyimide (SPI)
Download this article as:| Copy the following to cite this article: Sedesheva Y. S, Ivanov V. S, Wozniak A. I, Yegorov A. S. Proton-Exchange Membranes Based on Sulfonated Polymers. Orient J Chem 2016;32(5). |
| Copy the following to cite this URL: Sedesheva Y. S, Ivanov V. S, Wozniak A. I, Yegorov A. S. Proton-Exchange Membranes Based on Sulfonated Polymers. Orient J Chem 2016;32(5). Available from: http://www.orientjchem.org/?p=21266 |
Introduction
The advantage of fuel cells (FC), compared with other chemical current sources, consists in the possibility of a continuous supply of fuel and continuous current withdrawal for an unlimited period of time, as well as a higher coefficient of efficiency (COE) reaching 80% [1]. The main part of any fuel cell is the membrane-electrode assembly (MEA), consisting of the cathode and anode, which are separated by an electrolyte layer. One of the most promising electrolytes is proton-exchange (electrolytic) membrane based on polymers (PEM). Functions of fuel cell PEM based on the presence of the hydrophobic main chain, ensuring mechanical strength and residual sulfuric or phosphoric acid in the side chains to provide a proton transfer. The main task in the design of fuel cells is the selection of membrane materials with a high resistance towards chemical and physical degradation during operation, which leads to a monotonic decrease in the voltage on the plates of the fuel cell MEA and to reduction in its effectiveness to about 50% [2]. Thus ohmic losses at the membrane should be minimal, and the proton conductivity of the membrane should be high. Also, the membrane must have sufficient mechanical strength for the membrane electrode overmolding.
The main function of the proton-exchange membrane is the transfer of the proton to the cathode region, which is formed as a result of ionization of hydrogen at the anode, so for the high effective operation of fuel cell, the membrane should have a proton conductivity in the range of ~ 10-3to 10-1 S/ cm with a minimum of electronic conductivity. Sulfonated polymers effectively operate in the presence of water vapor, while the conductivity of phosphorylated polymers is less dependent on humidity. To increase conductivity, nanoscale oxides or solid electrolytes should be also introduced into the hydrophilic part [3].
During the operation of the fuel cell, water released at the cathode is partially absorbed by the membrane, leading to its swelling. This process is most noticeable when using humidified air. Excessive swelling of the membrane with its subsequent dryness with repeated activation/deactivation of the fuel cell is a highly undesirable process that leads to destruction and peeling of the thin active layer and deterioration of fuel cell characteristics.
Proton-exchange membrane also acts as the gas separation: it cuts off one anode side of the MEA, which contains hydrogen, from the cathode side, through which air or oxygen is blown-off. The low gas permeability is especially important when using hydrogen under increased pressure. Penetration of hydrogen to the cathode side is equivalent to the leakage current and should be minimized in order to increase the coefficient of efficiency of the fuel cell. The gas permeability of the membrane is less than 10-2ml / (min * cm2) is considered sufficient for long-term operation.
Chemical degradation of membrane polymers is caused by the action of hydrogen peroxide generated during fuel cell operation [4]. The presence of a trace of impurity cations Fe 2 +, Cu + in the membrane, catalyzes the dissipation of hydrogen peroxide molecules in order to form •OH-radicals, leading to attack of the polymer chains [5]. The intensity of peroxide membrane degradation is reduced by complete fluorination of the end groups of the macromolecules, by reduction of the degree of MEA pollution with Fe 2 +, Cu + cations, capable of catalyzing the formation of • OH-radicals and by the use of antioxidants.
Physical degradation of the membranes is associated with inelastic deformations, the formation of defects and reorganization of the polymer structure. Under the conditions of occurrence of creep, resulting in the thinning of the membrane and formation of defects, sulfonatedpolyarylenes show great stability. At the same time, the formation of microcracks and the local point defects in the membrane, increasing the transfer of gas reagents less typical for Nafion -type perfluorinated membranes.
Thus, a number of requirements are put forward for membrane characteristics, optimization which should be carried out as a whole, because of their close interrelation. Another important factor is the production cost of the finished membranes. Our review is devoted to the considerations of the various types of PEM used in fuel cells operating at temperatures up to 100° C, their main characteristics, advantages and disadvantages. The class of polyimide-based hydrocarbon proton-exchange membranes is separately considered as promising for widespread use in fuel cell, such membranes are of interest for our further experimental development.
Proton-exchange membranes based on polymers
Proton-exchange membranes based on polymers can be divided into two broad classes: polymeric electrolytes containing acid groups and composite membranes based on polymers. Let’s consider both of these classes in detail.
Proton-exchange membranes based on polymers containing acid groups
Perfluorinated membranes
Currently, the most common polymer membrane for fuel cells is perfluorinated ion-exchange membrane Nafion, developed by DuPont company in 1966, which is a copolymer of tetrafluoroethylene and a comonomer having side chains of perfluorinated vinyl ether with end sulfonate groups [6] (see Fig. 1). It is also known a number of analogs of Nafion – AsahiGlass Company products (Flemion), AsahiChemical (Aciplex), DowChemical, 3M, FuMA-Tech (Fumapem) and etc., differing minor variations in the structure of side chain of polymer molecule. [2].
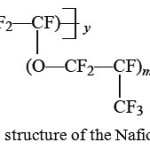 |
Figure 1: The structure of the Nafion membranes |
Materials based on these polymers are highly effective at operating temperatures up to 90°C [7]. The effectiveness of proton transfer therein is determined by the presence of moisture adsorbed from the atmosphere [6] because its own proton conductivity of such membranes is very small. This requires the introduction of additional devices and increases the cost of electrochemical generators. In addition, to prevent the poisoning of platinum electrodes with impurities of CO and hydrogen sulfide contained in the fuel, its pretreatment is required [2]. For such membranes, by varying the ratio between the two comonomers (see above), the ion-exchange capacity and equivalent weight of the polymer membrane depending on it can be varied (weight of the polymer, which contains 1 mole of sulfonate groups) [6]. The most widely used membranes are the membranes having an equivalent weight of 1000 and 1100 g / mol, which provides high proton conductivity and a satisfactory mechanical properties. The thickness of the membranes ranges from 50 to 250 microns at maximum proton conductivity of 0.2 to 0,05 S / cm at 30 ° C.
There are two models of proton transport in membranes of Nafion type (Fig. 2) [8].
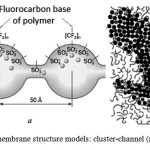 |
Figure 2: Nafion membrane structure models: cluster-channel (а) and cluster (b) |
According to the cluster- channel model of Gierke (Fig. 2a), the self-organization of polymer matrix with formation of clusters occurs in the membrane, these clusters are separated by narrow channels filled with water, through which the proton transfer is carried out by means of side chains with sulfonic groups faced to them. According to the channel model, membrane transport channels, saturated with water, and sulfonic groups are divided by hydrophobic polymer chains (Fig. 2b).
Proton conductivity of such membranes is carried out only in the presence of a significant amount of water absorbed (Fig. 3).
![Figure 3: The dependence of the proton conductivity of Nafion membrane-115 from moisture contents (the number of water molecules per one sulfonic acid group) [9]](http://www.orientjchem.org/wp-content/uploads/2016/09/Vol32No5_Prot_YULI_fig3-150x150.jpg) |
Figure 3: The dependence of the proton conductivity of Nafion membrane-115 from moisture contents (the number of water molecules per one sulfonic acid group) [9] |
Perfluorinated polymer sulfonic acids such as Nafion at temperatures greater than 90 ° C, lose water and cease to conduct protons. Increasing the degree of sulfonation allows to maintain an acceptable proton conductivity of the membranes to the high temperatures, but has a negative effect on their mechanical properties. To increase the operating temperature of the fuel cells, the following measures are applied: the increase in gaseous reactants pressure, the use of thin membranes, the generation of water in the membrane, the introduction of water presence regulators in the membrane and liquid or solid proton-exchange dopants [10].
Excessive increase in gaseous reactants pressure can increase the intensity of degradation processes and requires implementation of additional compressors system, which expended electric power during their operation, which reduces the efficiency of the plant. A serious disadvantage of perfluorinated polymer sulfonic acids is also their high price and high permeability in relation to methanol – for use direct methanol oxidation in fuel cell. In this regard, in recent years, the researchers pay much attention to various non-fluorinated or partially fluorinated hydrocarbon polymeric sulfonic acids, which may become a real alternative to Nafion [2]. Typical examples of such hydrocarbon polymers include sulfonatedpolyetheretherketones, polyarylene ethers, polysulphones, polyimides.
Hydrocarbon polymeric membranes including partially fluorinated
Unlike perfluorinated sulfonic acids, the synthesis of hydrocarbon polymers requires lower manufacturing costs, lower permeability to oxygen and methanol, lower sensitivity of the proton conductivity to the extent of reactants humidification and a better ability to retain water at high temperatures due to the lower hydrophobicity of macromolecules, which increases the possible temperature range of their operation. Nevertheless, hydrocarbon polymers show low conductivity and mechanical properties balance, because increasing the degree of sulfonation leads to loss of mechanical stability of the membranes in the presence of water. At equal degrees of sulfonation, the self-organizing proton-conducting channels in the hydrocarbon membranes are less signified, more sinuous and with less percolation [11].
A sulfonated polystyrene was proposed in 1960 as the hydrocarbon polymer membrane of the first generation, on the basis of which was created a generator that was used as a source of electricity and drinking water for astronauts with power of 1 kW. Work resource of installation was limited due to the chemical degradation of the membranes under the influence of peroxides formed on the cathode. The new generation of membranes of this type was partially fluorinated and was developed on the basis of α, β, β-trifluorostyrene known as BAM3G (Fig. 4).
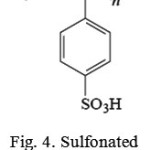 |
Figure 4: Sulfonated polystyrene |
These membranes have low equivalent weight ranging from 375 to 920 g/mol and a significantly higher degree of swelling as compared to Nafion membranes. When tested in a fuel cell, the BAM3G membranes show similar characteristics at low current densities and higher current densities of 0.6 A/cm2 (Fig. 5) [13].
![Figure 5: Current-voltage characteristics of fuel cells on Nafion membranes -117 (1), Dow (2) and BAM3G01 (3) [12].](http://www.orientjchem.org/wp-content/uploads/2016/09/Vol32No5_Prot_YULI_fig5-150x150.jpg) |
Figure 5: Current-voltage characteristics of fuel cells on Nafion membranes -117 (1), Dow (2) and BAM3G01 (3) [12]. |
Introduction of polystyrenesulfonic acid to side chains of the macromolecules is a common way of modifying the fluorinated polymer matrix and more cost-effective than the production of Nafion. The fluorine-containing materials can be modified using this way [14-18], among which polyvinylidene fluoride – vinylidene fluoride copolymer or tetrafluoroethylene with hexafluoropropylene with different molar ratio of comonomers [18-20]. The values of proton conductivity and permeability by hydrogen of resulted membranes with high degrees of inoculation are comparable to Nafion and reach 0.11 S/ cm at 100% humidity and room temperature [19-20]. A significant disadvantage of these membranes is their high degree of swelling, greatly exceeding the values for Nafion [19], and degradation during operation in the presence of peroxides, which significantly reduces the possibility of their use.
Polyetherketones (PEK), polyetheretherketone (PEEK), polysulfones (PS), polyethersulfones (PES) and polyarylethersulphones (PAES), polyphenylquinoxalines (PPQ), polyimides (PI) (Fig. 6), including partially fluorinated derivatives of these polymers have higher chemical and thermal stability, mechanical strength, and a large variety of possible chemical structures, compared with sulfonated polystyrenes. Introduction of sulfonic groups into these polymers is possible in two ways: by sulfonation of the finished polymer or by polymerizing sulfonated monomers. Regardless of the method of sulfonation, it is always necessary to optimize the sulfonic groups’ content in polymer, as the increase in their number significantly increases the degree of swelling and solubility of the polymer in water. It should be noted that the second way allows to avoid chain degradation and side reactions, because the chains are formed in the preparation of end films. Moreover, the method allows to precisely control and set the position and number of sulfonic groups in the polymer by the selection of structures and initial monomer ratios [21]
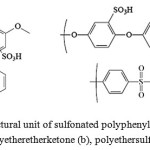 |
Figure 6: Structural unit of sulfonatedpolyphenylquinoxaline (a), polyetheretherketone (b), polyethersulfone (c) |
Membranes based on PPQ (Figure 6a.) BAM1G of varying thickness (40 – 120 microns) exhibit good mechanical properties in both dry and wet conditions, their characteristics are comparable with Nafion-117 with an equivalent weight of 390 – 420, however, work duration in the fuel cell until the degradation is low – an average of 350 hours [22].
There are commercially available forms such as Udel and Victrex respectively among developed PAES and PES (Fig. 6c). Ballard Advanced Materials Company has developed PEM under a trade name BAM2G being partially fluorinated PAES with varying degrees of sulfonation (it is impossible for PAES to get more than one sulfonic group in the monomer unit [23-26]). When tested in a fuel cell PAES insoluble in water show better performance compared to Nafion membranes -117, however, their lifespan is limited to about 500 hours. The proton conductivity of membranes based on sulfonated PES on Nafion level is achieved with sulfonation degrees about 90%, leading to high swelling degree which substantially increases with increasing temperature to 80 ° C, which in turn decreases the mechanical properties. Crosslinking the PES chains with diamine molecules results in reducing the degree of swelling and conductivity [24].
Introduction of sulfonic groups (only one per monomer unit) [27-33] into thermostable poorly soluble PEEK (fig. 6b) reduces the thermal stability up to 240 ° C and increases the solubility in organic solvents [34, 35], when the degree of sulfonation of more than 70%, PEEK becomes soluble in water. The water absorption of sulfonated PEEK with 65% degree of sulfonation and 100% humidity is 8%, the conductivity is about 10-5 S/ cm [36]. Reducing the degree of swelling of sulfonated PEEK by chemical crosslinking of macromolecules or adding agents forming strong hydrogen bonds [37, 38] leads to partial blocking of sulfonic groups and reduction of proton conductivity of membranes.
A new class of thermally and chemically stable polymers taken as promising for use as membranes [39], modified easily by introducing a variety of side chains are polyphosphazenes ( Fig. 7). Difficulties are associated with preparation of the water-insoluble polymers [40-45], and conditioning their hydrophile / hydrophobic properties. Water-insoluble membranes based on polyphosphazenes can be prepared by crosslinking, introduction of alkyl groups to the aromatic ring of the side chains or by varying the degree of sulfonation of the polymer [46].
![Figure 7: Structural unit of polyphosphazene: a - base polymer, b - sulfonated [41]](http://www.orientjchem.org/wp-content/uploads/2016/09/Vol32No5_Prot_YULI_fig7-150x150.jpg) |
Figure 7: Structural unit of polyphosphazene: a – base polymer, b – sulfonated [41] |
Proton-exchange membrane based on polyimides
The use of polyimides (Fig. 8) as the matrix to create a fuel cell membrane is based on the combination of their characteristics, such as thermal, mechanical, chemical resistance and the absence of electronic conductivity [47-53]. The presence of hydrophilic properties and the proton conductivity is attained by introducing of sulfonic groups into the main or side chains of the polyimide (Fig. 8 a, b).
![Figure 8: SPI types (a, b -phthalic type, c- naphthalic type) [54]](http://www.orientjchem.org/wp-content/uploads/2016/09/Vol32No5_Prot_YULI_fig8-150x150.jpg) |
Figure 8: SPI types (a, b -phthalic type, c- naphthalic type) [54] |
The first generation of membranes based on sulfonated polyimides (SPI) showed proton conductivity values in the range from 2 * 10-3 to 4 * 10-2 S/ cm [49,54,55], the next generation – up to 1,201 S / cm at 80 °C and 1.67 S/cm at 120 °C (for SPI composite / graphene material) that is comparable with Nafion type membranes. Water absorption and coefficient of osmotic resistance are insignificantly changed over a wide range of temperatures (Fig. 9). Membranes based on SPI exhibit a lower degree of degradation in activation/deactivation of TE, better retain water, have low permeability to methanol, especially at increased temperatures [56-58].
![Figure 9: Swelling - temperature dependence for SPI and Nafion 117 [75]](http://www.orientjchem.org/wp-content/uploads/2016/09/Vol32No5_Prot_YULI_fig9-150x150.jpg) |
Figure 9: Swelling – temperature dependence for SPI and Nafion 117 [75] |
The disadvantage of membranes based on SPI consists in the tendency of imide rings to hydrolysis under the operating conditions of the fuel cell, resulting in a decrease in the average length of the macromolecules after 200 hrs. of testing at 130 °C – almost by 4 times [59-65]. This negative effect, in turn, deteriorates the mechanical properties of the membrane, causing cracks and increased gas permeability and leads to shortened service life of the fuel cell.
At the first stage of hydrolysis, water molecules join the carbonyl carbon atom this process further leads to the destruction of the imide cycle and the main chain. (Fig. 10). Thus, electrophilicity of carbonyl carbon atom of the imide cycle directly affects the polyimides resistance to hydrolysis.
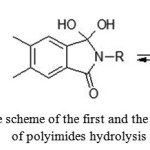 |
Figure 10: The scheme of the first and the second stages of polyimides hydrolysis |
Reducing the electrophilicity of carbonyl carbon atom of the imide cycle and thereby decrease in the activity of the hydration [66, 67] can be achieved in several ways: the transition from five-membered ring (phthalic type) to the six-membered ring (naphthalic type) (Fig. 8). [52,61,68 69]; introducing the bulky unit into the main or side chain of polyimide [70,71] and the electron-donating bridging sulfur atoms [72-74] phenoxy groups [75], benzophenone groups [76] into the main chain; the use of bi-naphthalene system (Fig. 11) [77, 78]; introducing sulfonic groups into the side chain rather than the main chain of the polyimide to reduce their electron-accepting effects [71, 79].
Since the hydrolysis reaction of imide rings is reversible [80] and the main chain of polyimide may be reduced in case of close distance between the units [81], the increase in the degree of crosslinking of the macromolecules facilitates the retention of frame and self-recovery of chains [82]. SPI with a higher degree of crosslinking can sorb more water with less degree of hydrolytic degradation [81, 83-85], which, in turn, increases the proton conductivity [86].
![Figure 11: SPI with Bi-naphthalene aromatic system in the main chain [41]](http://www.orientjchem.org/wp-content/uploads/2016/09/Vol32No5_Prot_YULI_fig11-150x150.jpg) |
Figure 11: SPI with Bi-naphthalene aromatic system in the main chain [41] |
Sorption of water by macromolecules of rigid-chain polymers significantly increases the bond stress in chains, and their tendency to destruction. Use of polyimides with flexible main chains can reduce the stress in water arising from matrix swelling, while the mechanical properties of the polymer remain unchanged in much larger quantities of absorbed water [87-89], however, the mobility of the main chain inhibits repeated recyclization of hydrolyzed imide cycles of polymer.
Membranes based on porous sponge-like non-sulfonatedpolyimides , pores of which were filled with the various SPI, demonstrated a higher hydrolytic stability than membranes prepared from the same SPI, but without the porous polyimide matrix [90].
Fuel cell was manufactured based on membranes from various SPI and tested. The tests showed that while a full operation of the fuel cell (namely, starting time of PEM degradation and reducing its efficiency) is strongly dependent on the temperature of its operation [91]. A fuel cell based SPI with sulfonic acid groups in the side chains, usually worked for a longer period of time than a fuel cell with sulfonic acid groups in the main chain, however, they worked worse than fuel cells based on Nafion [92,93].
Composite materials for polymer electrolytes
The manufacture of composite materials with nanoscale inorganic additives dispersed in a polymer matrix allows improving the mechanical properties, enhancing thermal stability up to 120 °C and reducing the dependence of electrotransport characteristics on humidity [94]. Proton-exchange polymers such as Nafion and sulfonated polystyrenes, polysulfones, polybenzimidazoles are often used as matrix.
Nanoscale oxides or salts, not generating protons, but fixedly retaining structurally related water or inorganic solid proton-exchange electrolytes – usually heteropolyacids, zirconium phosphate, or cesium sulfate are normally used as inorganic additives.
The use of thin (5-30 micron) composite membranes significantly enhances water balance by back diffusion and at high withdrawn currents (generating of large quantities of water) enables to maintain a relatively high degree of hydration in the membrane, providing the acceptable proton conductivity. The concept of “self-humidifying” [95] implies the injection of nanoscale platinum particles in combination with ultrafine particles of silicon and titanium oxides directly into the membrane thickness (Fig. 12). The function of the platinum particles consists in capture of molecular reagents penetrating through the membrane as a result of the transfer, and their conversion into the water. Oxide particles serve as the “reservoirs” for the generated water. Disadvantages of this approach consist in the increased use of the precious metal and the generation of peroxide radicals in the membrane thickness.
![Figure 12: Illustration of “self-humidifying” concept [95]](http://www.orientjchem.org/wp-content/uploads/2016/09/Vol32No5_Prot_YULI_fig12-150x150.jpg) |
Figure 12: Illustration of “self-humidifying” concept [95] |
It is safer in this respect to include superfine (nanoscale) hydrophilic particles in the membrane structure without electro-catalytic activity: oxides of silicon, aluminum, zirconium, titanium.
Excessive content of the inorganic phase may introduce fragility, although the mechanical properties of the resulting composite can be varied by using different types of silanes including a functional side groups (sulfonic groups), which may facilitate proton transport. Such membranes are considerably more efficient at high temperatures [94].
The conductivity of the hybrid membrane developed on the basis of Nafion / SiO2 system [95-98], has higher values than the unmodified Nafion, reaching 10-7 – 10-4 S/cm in the absence of moisture in the environment and at temperatures over 80 ° C, it decreases with increasing SiO2 content. In some cases, the composite membranes become “self-humidified” due to the back diffusion of water generated in the cathode region [99]. To improve the conductivity of such membranes it was suggested [100] to includenanoscale platinum into a polymer matrix, where oxidation of hydrogen with oxygen will occur to form water. Due to the low alcohol permeability of the composite membranes, and higher operating temperature, their use in fuel cells of direct methanol oxidation is very efficient, the specific power of the cell at 145 ° C and a voltage of 0.5 V can achieve up to 350 mA / cm2.
Introduction of other nanoscale oxides into the matrix of the proton-exchange polymers results in virtually the same results. Additives of titanium dioxide and tungsten trioxide [101] reduce the temperature of the membrane degradation. All oxides in the composition of a methanol fuel cell at 110 ° C and 70% RH (relative humidity) increase the membrane conductivity at reduced humidity in the range Nafion / SiO2>Nafion / WO3>Nafion / TiO2>Nafion (Fig. 13).
![Figure 13: SEM of recast composite membranes: (a) Nafion/SiO2, (b) Nafion/TiO2, (c) Nafion/WO3, and (d) Nafion/SiO2/PWA [101].](http://www.orientjchem.org/wp-content/uploads/2016/09/Vol32No5_Prot_YULI_fig13-150x150.jpg) |
Figure 13: SEM of recast composite membranes: (a) Nafion/SiO2, (b) Nafion/TiO2, (c) Nafion/WO3, and (d) Nafion/SiO2/PWA [101]. |
Nanocarbon materials are also used as additives to the Nafion proton-exchange membranes: a mixture of fullerene and fulerenol [102], carbon nanotubes [103]. Such membranes compared to the initial material more tightly retained water and showed higher conductivity under low humidity. Conductivity characteristics of composite membranes of 50 microns containing 1% of the nanotubes were similar to those of Nafion NRE-212, and the mechanical characteristics were considerably higher.
The systems containing zirconylphosphates and heteropoly compounds are stood out from among the polymers doped with crystalline solid electrolytes. Zirconium phosphate [104] and related layered structures exhibit high proton conductivity at room temperature (more than 10-2 S/cm) and maintain these values up to 300 ° C [105,106]. Introduction of such compounds into the proton-exchange matrix increases the thermal stability. Conductivity of Nafion / Zr (HPO4)2 system at 100 ° C and 100% humidity was 0.1 S/cm [107] and the SPEEK / Zr (HPO4)2 system under the same conditions was 0.01 S/cm [108 ]. Adding sulfonatedpolyetherketones, amorphous SiO2 particles and zirconium phosphate (sulfophenyl phosphate and zirconium phosphate) to the membranes’ composition resulted in an increase in proton conductivity at 100 ° C [109].
Heteropoly acids with Keggin’s anion of type H3PW12O40 • nH2O, H4SiW12O40 • nH2O and high water content at room temperature have the conductivity, which is very highly dependent on external conditions, since it is related to the presence of crystal water in the structure, which is easily dehydrated on lowering the ambient humidity or temperature increase. When using solutions of these acids in the fuel cells, the electrode – electrolyte boundary is much less sensitive to impurities of carbon monoxide in invading hydrogen [110]. Fuel cells derived from solid heteropolyacids [111] exhibited unsatisfactory mechanical properties. The administration of heteropoly compounds in the polymer matrix was more effective.
Composite membranes based on Nafion / heteropoly acid (H3PW12O40, H3RMo12O40, H4SiW12O40, H4SiMo12O40) systems with particles of several microns have high proton conductivity at temperatures above 100 ° C under conditions of low humidity [112]. Compounds containing molybdenum, were unstable to oxidation-reduction processes in the fuel cell, there was a gradual leaching of heteropoly compound. To stabilize the membranes, the part of the protons resulting from ion- exchange process, was replaced by ions of Cs +, NH4+, Rb+ and Tl+, that allowed to significantly reduce the leaching of heteropoly compounds from the system, the membranes had low permeability by hydrogen even at a thickness of 28 microns and their conductivity at 120 ° C and 35% RH was 1,6 • 10-2 S / cm. The membrane electrode assembly of hydrogen-air fuel cell, created based on the system stabilized by Cs +, showed good characteristics at 120 ° C and 35% RH [113].
Conductivity of optimized [114] composition of composite membranes of Nafion / SiO2 / H3PW12O40 system was better than that of Nafion-117, higher efficiency compared with the undoped membranes. Hybrid membranes Nafion / SiO2, doped with phosphotungstic acid and silicotungstic acids may operate at 145 ° C in fuel cells based on methanol [111].
Conclusion
The need to develop technologies of material preparation with enhanced proton conductivity, heat resistance and resistance to aggressive media, to create membranes of fuel cells remains pertinent. Despite the fact that polyfluorosulfonic acids (Nafion and analogs thereof) are still superior membrane materials for fuel cells. Despite the fact that these systems operate at a relatively low temperature of 100 ° C and quickly reach the operating power, they still have several significant drawbacks, which currently prevent expansion of fuel cell as a viable alternative to generators operating on hydrocarbon fuels.
Such polymers as polyetherketones, polyetheretherketones, polysulfones, polyethersulfones currently are particularly attractive for development of proton-exchange membrane of fuel cells.
Due to a combination of unique properties such as high proton conductivity, mechanical and hydrolytic stability, low permeability to gases and liquids and relatively low cost compared to perfluorinated materials of Nafion type, SPIs are excellent equivalents used as a matrix candidates to create materials for membranes on their basis, including the block structure.
Applied research carried out with the financial support of the government represented by the Ministry of Education of the Russian Federation under the Grant Agreement No.14.625.21.0036 dated October 27, 2015. (Unique identifier of applied research (project) RFMEFI62515X0036).
References
- Carrette L.; Friedrich К. A.; Stimming U. Fuel Cells – Fundamentals and Applications. 2001; 1; 1; 5-39.
- Qi Z. Proton Exchange Membrane Fuel Cells. CRC Press. 2013; 371.
- Borup R.; Meyers J.; Pivovar B.; Kim Y. S.; Mukundan. R.; Garland N.; Myers D.; Wilson M.; Garzon F.; Wood D.; Zelenay P.; More K.; Stroh K.; Zawodzinski T.; Boncella J.; McGrath J. E.; Inaba M.; Miyatake K.; Hori M.; Ota K.; Ogumi Z.; Miyata S.; Nishikata; A.; Siroma Z.; Uchimoto Y.; Yasuda K.; Iwashita N. Journal of Electrochemistry Chem. 2007, 107, 3904–3951.
- Schiraldi D. A. J. Macromol. Sci. C: Polym. Rev. 2006, 46, 315–327.
- Nagappan R.; Nazih H.; Sangeev M. ElectrochimicaActa. 2008, 53, 3279-3295.
- Web res.: www.pemeas.com
- Kreuer K.D. J. Membrane Sci. 2001, 185, 1, 29-39.
- Zawodzinski T.A. Jr.; Derouin C.; Radzinski S.; Sherman R.J.; Smith V.T.; Springer Т.Е.; Gottesfeld S. J. Electrochem Soc. 1993, 140, 4, 1041-1047.
- Li Q.; He R.; Jensen J. O.; Bjerrum N. J. Chem. Mater. 2003,15, 4896–4915.
- OetjenH.‐F.;Schmidt V.M.;StimmingU.;Trila F.J. Electrochem. Soc.1996,143, 12,3838-3842
- Rikukawa M.; Sanui K. Prog.Polym. Sci. 2000, 25, 1463-1502.
- Brack H.P.; Buhrer H.G.; Bonorand L.; Scherer G.G. J. Mater. Chem.2000,10, 1795-1803.
- Lehtinena T.; Sundholma G.; Holmbergb S.; SundholmcF.; Björnbomd P.; Burselld M. Electrochim. Acta. 1998,43, 1881-1890.
- Brack H.P.; Wyler M.; Peter G.; Scherer G.G. Journal of Membrane Science. 2003,214, 1, 1–19.
- Grafting of pre-irradiated poly(ethylene-alt-tetrafluoroethylene) films with styrene: influence of base polymer film properties and processing parameters // J. Mater. Chem. 2000,10, 1795-1803.
- Nasef M.; Saidi H.; Yarmo M.A. J. New Mat. Electrochem. Syst. 2000,3, 309.
- Wang A.A.; Capuano G.A. J. Electrochem. Soc. 1998,145, 781.
- KallioТ.;Lundstrom M.; Siindholm G. J. Appl. Electro-chem. 2002,32, 11-18.
- Gebel G.; Aldebert P.; Pineri M. Polymer. 1993,34, 333-339.
- Noshay A.; Robeson L.M. J. Appl. Polym. Sci. 1976,20, 1885.
- Johnson В.C.; Yilgor I.; Tran C. J. Polym. Sci.: Polym. Chem. Ed. 1984,22, 721.
- Genova-Dimitrova P.; BaradieВ.;Foscallo D. J. Membrane Sci. 2001, 185, 59.
- Nolte R.; Ledjeff K; Bauer M.; Mulhaupt R. Journal of Membrane Science. 1993,83, 211-220.
- Kaliaguine S.; Mikhailenko S. D.; Wang K,Catal. Today. 2003,82, 213.
- Robertson G,;Mikhailenko S. D.; Wang K. J. Membrane Sci. 2003, 219, 113.
- Roziere J.; Jones D. J. Annu. Rev. Mater. Res. 2003,33, 503-555.
- Bauer В.; Jones D. J.; Roziere J. J. New Mater. Electro-chem. Syst. 2000, 3, 93-98.
- Zaidi S. M. J.; Mikhailenko S.D.; Robertson G.P. J. Membrane Sci. 2000, 173, 17-34.
- Bishop M. Т.;Karasz F.E.; Russo P.S.; Langley К.H. Macromolecules. 1985,18, 86-93.
- Linkous C.A.; Anderson H.R.; Kopitzke R.W. Development of new proton exchange membrane electrolytes for water electrolysis at higher temperatures // Nelson G.L. In: Proc. of the 11th hydrogen conf. 1996, 559.
- Bailey C.; Williams D.J.; Karasz F.E.; Macknight W.J. Polymer. 1987,28, 1009-1013.
- Kerres J.; Cui W.; Reichle S. J. Polym. Sci. Part A Polym. Chem. 1996, 34, 2421-2438.
- Wnek G.E.; Rider J.N.; Serpico J.M. New hydrocarbon proton exchange membranes based on sulfonated styrene-ethylene/butylene-styrene triblock copolymers // In: Proc. of the First Int. Symp. on Proton Conducting Membrane Fuel Cells; The Electrochemical Society Proceedings. 1995,95 (23). 247.
- Huang R. Y. M.; Shao P.; Burns С. M.; Feng X. J. Appl. Polym. Sci.; 2001,82, 2651-2660.
- Al-Omran A.; Rose J. B. Polymer. 1996, 37, 1735-1743.
- Kobayashi T.; Rikukawa M. Solid State Ionics. 1998,106, 219-225.
- Katime I.A.; Iturbe C.C. In: Polymeric Materials Encyclopedia, 5 (H-L). Boca Raton. FL CRC Press. 1996, 3097-3106.
- Cui W.; Kerres J.; Eigenberger G. Sep. Purif. Technol. 1998,14, 145-154.
- Kraytsberg A.; YairEin-Eli A. Energy Fuels. 2014,28, 12, 7303–7330.
- Wycisk R.; Pintauro P.N. J. Membrane Sci. 1996, 119, 155-160.
- Allcock H.R.; Fitzpatrick R.J.; Salvati L. Chem. Mater. 1991,3, 1120-1132.
- Allcock H.R.; Klingenberg E.H.; Welker M.F. Macromolecules. 1993,26, 5512-5519.
- Monotoneri E.; Gleria M.; Ricca G.; Pappalardo G.C. Macromol. Chem. 1989,190, 191.
- Allcock H.R. Soft Matter. 2012,8, 7521-7532.
- Gruzd A.S.; Trofimchuk E.S.; Nikonorova N.I.; Nesterova E.A.; Meshkov I.B.; Gallyamov M.O.; Khokhlov A.R. Int. J. Hydrogen Energy. 2013,38, 10, 4132–4143.
- Kondratenko M.S.; Ponomarev I.I.; Gallyamov M.O.; Razorenov D.Y.; Volkova Y.A.; Kharitonova E.P.; Khokhlov A.R. Beilstein J. Nanotechnol. 2013,4, 481–492.
- Hickner M.A.; Ghassemi H.; Kim Y.S.; Einsla B.R.; McGrath J.E. Chem. Rev.2004,104, 4587-4612.
- Zhang H.; Shen P.K. Chem. Rev. 2012,112, 2780.
- Cao Z.; Cao X.; Sun L.; He Y. Adv. Mat. Res. 2011, 239, 3032.
- Malay K. Ghosh and K.L. Mittal (Eds.) in Polyimides: fundamentals and applications. NY.: Marcel Dekker. Inc. 1996.
- Okamoto K.I. J. Photopolymer Sci. Tech. 2003,16, 247-254.
- Genies C.; Mercier R.; Sillion B.; Petiaud R.; Cornet N.; Gebel G.; Pineri M. Polymer. 2001,42, 5097-5105.
- Faure S.; Pineri M.; Aldebert P.; Mercier R.; Sillion B. US Pat 6245881. 1996.
- Miyatake K.; Asano N.; Watanabe M. Journal of Polymer Science Part A: Polymer Chemistry. 2003, 41, 24, 3901–3907.
- Woo Y.; Oh S.Y.; Kang Y.S.; Jung B. J. Membrane Sci. 2003, 220, 31-45.
- Miyatake K.; Zhou H.; Uchida H. Watanabe M. Chem. Commun. 2003, 368-369.
- He Y.; Tong C.; Geng L.; Liu L.; Lu C. J. Membrane Sci. 2014, 458, 36-46.
- Yin Y.; Yamada O.; Tanaka K. Okamoto K.-I. Polymer. 2006, 38, 197-219.
- Peinemann K.-V.; Nunes S.P.; Membrane Technology; Membranes for Energy Conversion. / John Wiley & Sons. Weinheim. 2008.2
- De Iasi R.; Russell J. J. Appl. Polym. Sci. 1971,15, 2965-2974.
- Jang W.; Lee C.; Sundar S.; Shul Y.G.; Han H. Polymer Degradation and Stability. 2005,90, 431-440.
- Aoki M.; Asano N.; Miyatake K.; Uchida H.; Watanabe M. J. Electrochem. Soc. 2006,153, 1154-1158.
- Chen X.; Chen K.; Chen P.; Higa M.; Okamoto K.-I.; Hirano T. J. Polym. Sci. A. Polym. Chem. 2010,48, 905-915.
- Yan J.; Liu C.; Wang Z.; Xing W.; Ding M. Polymer. 2007,48, 6210-6214.
- Rehman S.; Li P.; Zhou H.W.; Zhao X.G.; Dang G.D.; Chen C.H. Polymer Degradation and Stability. 2012,97, 1581-1588.
- Savadogo O. J. New Mater. Electrochem. Syst. 1998,1, 47.
- Genies C.; Mercier R.; Sillion B.; Cornet N.; Gebel G.; Pineri M. Polymer. 2001,42, 359-373.
- Asano N.; Miyatake K.; Watanabe M. J. Polym. Sci. A. Polym. Chem. 2006,44, 8, 2744–2748.
- Asano N.; Aoki M.; Suzuki S.; Miyatake K.; Uchida H.; Watanabe M. J. Am. Chem. Soc. 2006,128, 1762-1769.
- Wei H.; Chen G.; Cao L.; Zhang Q.; Fang Q.Y.; Fang X. Mater. Chem. A.2013, 1, 10412 – 10421.
- Wei H.; Fang X. Polymer. 2011,52, 2735-2739.
- Zhang F.; Li N.; Cui Z.; Zhang S.; Li S. J. Membrane Sci.2008, 314, 24.
- Zhang F.; Li N.; Zhang S.; Li S. J. Power Sources. 2010,195, 2159-2165.
- Zhang F.; Li N.; Zhang S. J. Appl. Polym. Sci. 2010,118, 3187-3196.
- Li N.; Cui Z.; Zhang S.; Li S.; Zhang F. J. Power Sources. 2007,172, 511-519.
- Yan J.; Huang X.; Moore H.D.; Wang C.-Y.; Hickner M.A. Int. J. Hydrogen Energy. 2012, 37, 6153-6160.
- Yin Y.; Fang J.; Watari T.; Tanaka K.; Kita H.; Okamoto K.-I. J. Mater. Chem. 2004,14, 1062-1070.
- Perrot C.; Gonon L.; Marestin C.; Gebel G. J. Membrane Sci. 2011, 379, 207-214.
- Marestin C.; Gebel G.; Diat O.; Mercier R. Sulfonated Polyimides in Advances in Polymer Science. / Fuel cells II; G. G. Scherer (Ed.); Springer. 2008, 216,185.
- Sundar S.; Jang W.; Lee C.; Shul Y.; Han H.J. Polym. Sci. B Polym. Phys. 2005,43, 2370-2379.
- Jin R.; Li Y.; Xing W.; Qiu X.; Ji X.; Gao L. Polym. Adv. Technol. 2012,23, 31-37.
- Lin C.-C.; Chang C.-B.; Wang Y.-Z. J. Power Sources. 2013,223, 277-283.
- Sung K.A.; Kim W.-K.; Oh K.-H.; Choo M.-J.; Park J.-K. J. Korean Electrochem. Soc. 2009,12, 245-250.
- Belkhiri Z.; Zeroual M.; Moussa B. H.; Zitouni B. Revue des EnergiesRenouvelables. 2011,14, 121.
- Einsla B.R.; Kim Y.S.; Hickner M.A.; Hong Y.-T.; Hill M.L.; Pivovar B.S.; McGrath J.E. J. Membrane Sci. 2005, 255, 141-148.
- Fang J.; Guo X.; Harada S.; Watari T.; Tanaka K.; Kita H.; Okamoto K.-I. Macromolecules. 2002,35, 9022-9028.
- Guo X.; Fang J.; Watari T.; Tanaka K.; Kita H.; Okamoto K.-I. Macromolecules. 2002,35, 6707-6713.
- Wang T.; Sun F.; Wang H.; Yang S.; Fan L. Polymer. 2012,53, 3154-3160.
- Meyer G.; Gebel G.; Gonon L.; Capron P.; Marscaq D.; Marestin C.; Mercier R. J. Power Sources. 2006,157, 293-301.
- Kabasawa A.; Saito J.; Yano H.; Miyatake K.; Uchida H.; Watanabe M. Electrochim. Acta. 2009,54, 1076-1082.
- Kabasawa A.; Saito J.; Miyatake K.; Uchida H.; Watanabe M. Electrochim. Acta. 2009,54, 2754-2760.
- Wycisk R.; Pintaura P.N.; Wang W.; O’Connor S. Polyphosphazeneionexchange membranes // Poly-phosphazeneionexchange membranes. Proc. North American membrane society 7th annual meeting. 1995.
- Uchida H.; Ueno Y.; Hagihara H.; Watanabe M. J. Electrochem. Soc. 2003,150, 1, A57–A62.
- Adjemian K. T.; Lee S. J.; Srinivasan S.; Benziger J.; Bocarslya A. B. J. Electrochem. Soc. 2002,149, 3, A256–A261.
- Mauritz. KA. Mater. Sci. and Eng. C. 1998, 6, 121-133.
- Jung D. II.; Cho S. Y.; Peck D. H. J. Power Sources. 2002,106, 173-177.
- Adjemian К. Т.;Srinivasan S.; Benziger J.; Bocarsly A. B. Journal of Power Sources. 2002,109, 356-364.
- Linag Z.X.; Zhao T.S.; Prabhuram J. J. Membr. Sci. 2006, 283, 219-224.
- Wang H.T.; Holmberg B.A.; Wang Z.B. J. Mater. Chem. 2002,12, 834-837.
- Hagihara H.; Uchida H.; Watanabe M. Electrochim. Acta. 2006,51, 3979-3985.
- Zhi-Gang Shao; HongfengXu; Mingqiang Li; I-Ming Hsing. Solid State Ionics. 2006,177, 779-785.
- Tasaki K.; DeSousa R.; Wang H. J. Membrane Sci. 2006,281, 570-580.
- Yong-Hao Liu; Baolian Yi; Zhi-Gang Shao.Electrochem. Solid-State Lett. 2006,9, A356-A359.
- Clearfield A.; Smith J. J. Inorg. Chem. 1969,8, 431-436.
- Alberti G.; Casciola M.; Costantino U.; Vivani R. Adv. Mater. 1996,8, 291-303.
- Alberti G.; Casciola M. 16 Phosphates and phosphonates of tetravalent metals as protonic conductors // In: Proton Conductors; Solid; Membranes and Gels; Materials and Devices. Ed. Ph. Colombian. 1992.
- Costamagna P.; Yang C.; Bocarsly А. В.;Srinivasan S. Electrochim. Acta. 2002,47, 1023-1033.
- RuffmannВ.; Silva H.; Schulte В.; Nunes S.Solid State Ionics. 2003,162-163, 269-275.
- Hogarth W.H. J.; Diniz da Costa J.C.; Lu G.Q.(Max). J. Power Sources. 2005,142, 223-237.
- Giordano N.; Staiti P.; Hocevar S.; Aricm A. S.Electrochim. Acta. 1996,41, 397-403.
- Staiti P.; Hocevar S.; Giordano N. Int. J. Hydrogen Energy. 1997,22, 809-814.
- Ramani V.; Kunz H.R.; Fenton J.M. J. Membrane Sci. 2004, 232, 31-44.

This work is licensed under a Creative Commons Attribution 4.0 International License.









