Polyporphyrin Complexes of Some Transition Metals. Synthesis and Catalytic Properties
A.V. Shakhvorostov1, Z. B. Atabekova1*, B. S. Selenova1, G. Zh. Yeligbayeva1 and G. I. Dzhardimalieva2
1Kazakh National Research Technical University named after K. I. Satpayev, Almaty, 050000, Kazakhstan.
2The Institute of Problems of Chemical Physics RAS, Chernogolovka, Moscow Region, 142432, Russian Federation.
Corresponding Author E-mail: zau888@mail.ru
DOI : http://dx.doi.org/10.13005/ojc/320517
The paper presents the results of synthesis of polyporphyrin structures and metal complex catalyzers at their basis. Porphyrin to be derived from the addition reaction of pyrrole and formaldehyde. Metal complex catalyzers to be derived at the reaction of complex formation of ions of Mn2+, Co2+, Ni2+ and Fe3+ with porphyrin. The structure, physical and chemical properties of derived materials to be examined with IR spectroscopy, differential thermal analysis, thermogravimetric analysis, scanning electron microscopy investigation. Catalytic activity of synthesized catalytic systems to be established at the reaction of decompounding of hydrogen peroxide and alkylaromatics oxidation by hydrogen peroxide. The processes have been conducted under soft conditions, and also at different organic solvents.
KEYWORDS:Porphyrin; metal complex catalyzers; hydrogen peroxide; oxidation; alkylaromatics
Download this article as:| Copy the following to cite this article: Shahvorostov A. V, Atabekova Z. B, Selenova B. S, Yeligbayeva G. Z, Dzhardimalieva G. I. Polyporphyrin Complexes of Some Transition Metals. Synthesis and Catalytic Properties. Orient J Chem 2016;32(5). |
| Copy the following to cite this URL: Shahvorostov A. V, Atabekova Z. B, Selenova B. S, Yeligbayeva G. Z, Dzhardimalieva G. I. Polyporphyrin Complexes of Some Transition Metals. Synthesis and Catalytic Properties. Orient J Chem 2016;32(5). Available from: http://www.orientjchem.org/?p=22659 |
Introduction
Metal porphyrins are wide spread in Nature and are the part of the whole range of enzymes, which are the effective catalyzers of hydrocarbon oxidation reactions. The actual task is creation of industrially important oxidation processes for n-alkanes and aromatic hydrocarbons of catalytic systems on the basis of porphyrin compounds, operating under soft conditions. [1,2]. Starting the late 70s of XX century the vast number of porphyrin compounds have been synthesized and examined at oxidation reactions of different substrates [3-8].
The main disadvantage of the systems is their relatively low stability, whereas during the reaction the porphyrin ligand itself is to decompose. For purposes of heterogenization of soluble metal porphyrins they can be immobilized at the surface of polymer or mineral carrying agent [9-13]. At papers [14,15] for heterogenization of metal porphyrins a specific approach to be used, involving the polymerization of porphyrin fragments. Polymer metal porphyrin to be effectively catalyzed hexahydrobenzene oxidation by iodobenzene.
Therefore, synthetic metal porphyrin complexes are appeared to be high-selective catalyzers of oxidation functionalization of different classes of hydrocarbons. Thereat fixation of synthetic metal porphyrin complexes at the surface of polymer or mineral carrying agents may lead to derivation of stable catalyzers saving selectiveness and catalytic activity.
Materials and Methods
The following materials were used for experimental studies:
Reagents
– Formaldehyde CH2O (99,5%) (Sigma-Aldrich);
– Pyrrole C4H5N (99,5%) (Sigma-Aldrich) was used without further purification;
– HCl hydrochloric acid (GOST 3118-77) as a catalyst, was used without further purification.
Inorganic Salts
– Manganese chloride MnCl2 * 4H2O brand “chemically pure” (JSC “Reahim”);
– Ferrous sulphate FeSO4 * 7H2O brand “chemically pure” (JSC “Reahim”);
– Cobalt nitrate Co (NO3) 2 * 6H2O brand “chemically pure” (JSC “Reahim”);
– Nickel nitrate Ni (NO3) 2 * 6H2O brand “chemically pure” (JSC “Reahim”).
All inorganic salts were further recrystallized from aqueous solutions.
Alkylaromatic Substrates
– Cumene S9N12 brand “chemically pure” (JSC “Reahim”);
– Toluene S7N8 brand “chemically pure” (JSC “Reahim”);
– Ethylbenzene S8N10 brand “chemically pure” (JSC “Reahim”).
Alkylaromatic substances were further distilled under vacuum.
Inorganic Solvents
– Bidistilled deionized water of H2O (BDV) (the resistance of 18 megohms).
Before using water the degassing process was carried out to remove dissolved air.
Organic Solvents
– Dichloromethane C2H4Cl2 (DCM) (99,9%) (Sigma Aldrich);
– Dimethyl C2H6OS (DMSO) (99,9%) (Sigma Aldrich);
– Dimethylformamide C3H7NO (DMF) (99,9%) (Sigma Aldrich);
– C6H6 benzene brand “chemically pure” (JSC “Reahim”) was further distilled under vacuum.
Original porphyrin (P) is derived on classic methodology [16], by the addition reaction of pyrrole and formaldehyde. At three-necked round-bottomed flask, equipped with back-flow condenser and offset for supply of inert gas, to a solution of 0.14 mole (4.204 g) of formaldehyde at 100 ml of boiled bidistilled deionized water to be added 0.5 ml (0.595 g) of hydrochloric acid (37%). Further to be added 0.7 mole (46.963 g) of pyrrole conducted dropwise, maintaining an interval in time for previous drop fully stirred at the value. After the last portion of pyrrol to be added, the suspension to be brought to a boil on a heating plate and boiled within 40 minutes. After 40 minutes passed the heating to be terminated and the suspension had been left to get cool in the open air down to room temperature. During the cooling process the brown crystals to be dropped out. Afterwards, the brown crystals to be filtered at Buchner funnel and left to dry under vacuum overnight. The experiment was conducted at the atmosphere of argon inert gas. After the reaction to be done, unwatered product represents the brown powder (the mass equals 11.829 g). Afterwards the product additionally to be irrigated by some amount of hot solution of dichloromethane. After the extraction the pure porphyrin to be derived. The porphyrin synthesis scheme is shown in Figure 1.
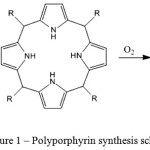 |
Figure 1: Polyporphyrin synthesis scheme |
At the table 1 are shown the data on polyporphyrin synthesis.
Table 1: Porphyrin synthesis conditions
|
М (pyrrole), g |
М (formaldehyde), g |
М (НСl), g |
Т,
|
τ, h |
М (polyporphyrin), г |
Yield, % |
|
46.963 |
4.204 |
0.595 |
100 |
1 |
9.246 |
68 |
For polyporphyrin metal complexes synthesis (Ме-PP) to a solution of 0.0016 mole of porphyrin at 100 ml DMSO added with 0.008 mole of salt of metals Fe(III), Co(II), Ni(II) and Mn (II). The mixture was brought to reflux and maintained for 3 hours. After the time ran out DMSO to be evaporated under vacuum. Derived precipitation to be washed under hot water for dissolving of unreacted MnCl2. Filtered precipitation to be unwatered within 24 hours under vacuum at temperature 50оС. The derived product does not dissolve at solvents common for mono-porphyrin derivatives. Obviously, during metal porphyrin complexes synthesis appears the formation of compound presenting oligomeric polymer chain consisting of porphyrin rings.
The process of formation of metal complexes appears by means of reaction of addition of divalent ion of metal to inner ring consisting of four pyrrole fragments (Figure 2). Continuous lines show metalorganic bonds, dashed lines show coordination interactions of nitrogen and metal atoms.
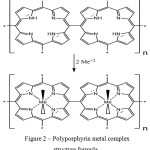 |
Figure 2: Polyporphyrin metal complex structure formula |
For the research of structure and physical properties of obtained materials the following physical and chemical methods were used.
FT-IR Spectroscopy (IR)
IR spectra were recorded on a spectrophotometer Carry 660 Agilent (USA) at a temperature of -25 ℃. A sample weighing 5 mg is placed in a mortar with 150 mg of potassium bromide (KBr) then milled until uniform in color and size of the mass of particles. The resulting mass is filled in a special pressing device between two polished metal plates that connect the vacuum pump, created a vacuum of 25 mbar. Special hydraulic press was pumped effort equal ≈9,5 T. Maintained sample under pressure for 6 ~ 7 minutes, then the resulting pellet was removed and placed in a special holder. The scanning speed was 1 cm-1/sec, a scan range from 650 to 4000 cm-1. The number of background scans was 8 times, the test sample 24 times.
Differential Thermal Analysis and Termogravimetric Analysis (DTA-TGA)
Studies of thermal stability, and thermogravimetric measurement samples over a wide operating temperature range (40 ° C to 700 ° C) were carried out on LabSys evo (France). Temperature set rate was 10 ° C / min, weight of sample 15 ~ 30 mg. The sample was placed in a cuvette of aluminum oxide (Al2O3) with a tight lid and mounted between the thermocouple unit.
X-ray Diffraction (XRD)
The crystal structure of the obtained metal complex catalysts Me-PP was investigated on the X-ray diffractometer X,Pert MPD PRO PANalytical (Holland) at a temperature T = 25 ℃, the mass of the sample ranged from 15-30 mg.
Scanning Electron Microscopy (SEM)
Particle size and surface structure of the particles were evaluated by using low vacuum scanning electron microscope JEOLJSM-6490LA (Japan). Voltage 15 kV electron gun, a residual pressure of 60 Pa.
Results and Discussion
The structure and properties of derived metal porphyrin complexes to be examined by aforecited physical and chemical methods. The data on gravimetric measurements of derived metal complexes is shown at the table 2.
Table 2: Data on gravimetric measurements of porphyrin metal complex
|
Metal complex |
Mass, g |
δ, g* |
σ, %** |
ω , %*** |
|
Mn-PP |
3.1018 |
0.1018 |
19.15 |
3.39 |
|
Fe-PP |
3.2862 |
0.2862 |
53.00 |
9.54 |
|
Co-PP |
3.2720 |
0.2720 |
47.87 |
9.06 |
|
Ni-PP |
3.2993 |
0.2993 |
52.51 |
9.98 |
*– polyporphyrin mass change; **– yield of reaction of complex formation compared to the theoretically possible one; ω – metal weight content at catalyzer.
Over the gained data the conclusion to be made that the reaction of complex formation in case of ions of Mn2+ goes weaker than with Fe2+, Co2+, Ni2+. This is due to the fact that for manganese (II) is less common to have complex formation, than for other d-elements. Obviously, in that case the formed complex compounds of manganese with pyrrole fragments are insufficiently stable, presence of which is limited by definite value of pH of solution.
The Figure 3 shows IR spectrum of original porphyrin (1) and Ме-PP complexes (2-5). The wide absorption brand at 3392 cm-1 for porphyrin (1) corresponds the secondary and tertiary atoms of nitrogen, this wide peak persists at metal complexes (2-5) at absorption brands 385, 3389, 3371 and 3344 cm-1 respectively. Displacement of absorption peaks to the side of low-frequencies oscillations corresponds the nitrogen atoms conjugation with atoms of metal. The absorption brands within the range of 1635 cm-1 for porphyrin (1) and 1634, 1629, 1614, 1622 cm-1 for Mn-PP, Fe-PP, Co-PP, Ni-PP (2-5) corresponds to oscillations at pyrrole ring, and also subtle oscillations within the range of absorption at 1700 cm-1.
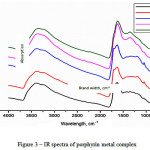 |
Figure 3: IR spectra of porphyrin metal complex |
The Figure 4 shows thermogravimetric data of synthesized metal porphyrin complexes. The absence of phase transitions of the first and the second type (absence of the peaks at the curves) within the interval of temperatures 50 – 150, and also insignificant change of the mass of the samples shows the stability of metal porphyrin complexes. At further heating within 150 – 425 smooth change of mass to be presented to the side of decrease, obviously, it to be induced by gradual decomposition of metal complexes. At temperatures higher than 450 the destruction of Ме-PP is about to happen.
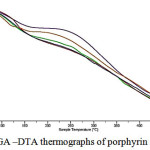 |
Figure 4: TGA –DTA thermographs of porphyrin metal complex |
A – original porphyrin, B – Ni-PP, C – Co-PP, D – Fe-PP, E – Mn-PP
Over the derived data X-ray phase diffraction analysis (Figure 5) the conclusion to be made that during the process of Ме-PP metal complex formation the main structure to be represented as amorphous part.
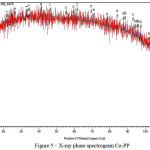 |
Figure 5: X-ray phase spectrogram Co-PP |
The surface of synthesized metal porphyrins represents the grain structure with highly developed system of microscopic pores (Figure 6 a,b).
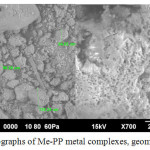 |
Figure 6: SEM photographs of Ме-PP metal complexes, geometric sizes and porosity |
Synthesized metal porphyrins as heterogenic catalyzers to be tested at the alkylaromatics oxidation reaction by hydrogen peroxide. The alkylaromatics oxidation reaction to be conducted at steel autoclave at atmospheric pressure. The process to be conducted at acetonitrile media. The hydrogen peroxide to be considered the preferable oxidizer at porous systems, as peroxide compounds are very mobile in the pores due to their smaller size. Therewith, the reagents are relatively cheap and environmentally safe. This system can be used in industrial and commercial scale. Nonetheless, aerobic oxidation, as expected, persists with lower speed because of inability of molecular oxygen to transfer into activated form comparing with highly active form, such as peroxides. Catalytic character of oxidation to be approved by the conduction of oxidation reaction in absence of any other catalyzer. As it turned out, the hydrogen peroxide is unable to oxidize the substrates in significant amount alone, it suggests that the reactions can persists only when the catalyzer presents at the system and facilitates the lowering activation energy of oxidation reaction.
The results of the experiments on cumene, toluene and ethylbenzene oxidation at metal complex catalyzers Mn-PP, Fe-PP, Co-PP, Ni-PP at the temperature 70 are shown at the table 3.
Table 3: Results of Alkyloramatic substrates oxidation reaction by hydrogen peroxide at metal porphyrin catalyzers at 70
|
Catalyzer |
Substrate |
Oxidizer |
Conversion, % |
|
Mn-PP |
cumene |
Н2О2 |
28.6 |
|
Mn-PP |
toluene |
Н2О2 |
17.7 |
|
Mn-PP |
ethylbenzene |
Н2О2 |
43.4 |
|
Fe-PP |
cumene |
Н2О2 |
33.3 |
|
Fe-PP |
toluene |
Н2О2 |
20.4 |
|
Fe-PP |
ethylbenzene |
Н2О2 |
26.5 |
|
Co-PP |
cumene |
Н2О2 |
36.1 |
|
Co-PP |
toluene |
Н2О2 |
14.9 |
|
Co-PP |
ethylbenzene |
Н2О2 |
49.7 |
|
Ni-PP |
cumene |
Н2О2 |
21.2 |
|
Ni-PP |
toluene |
Н2О2 |
6.5 |
|
Ni-PP |
ethyl benzene |
Н2О2 |
16.8 |
Over gained results the diagram to be done for metal complex catalyzers activities – Figure 7. The most active at cumene oxidation is Со-PP, at toluene oxidation is Fe-PP, at ethyl benzene is Mn-PP. Thereat Ni-PP at all reactions is the least active.
During cumene oxidation metal complex catalyzers mainly form up the mixture consisting of phenol and acetone (1:1). During toluene oxidation manly forms up benzyl alcohol and a small amount of benzaldehyde and benzoic acid. In case of ethyl benzene there to be formed up the mixture of methyl phenyl carbinol and methyl phenyl ketone.
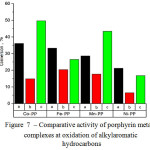 |
Figure 7: Comparative activity of porphyrin metal complexes at oxidation of alkylaromatic hydrocarbons |
For stability examination of synthesized metal porphyrins at oxidation reaction for aromatic hydrocarbons by hydrogen peroxide there to be conducted series of experiments at the same sample of catalyzer charge. Consecutive experiments on ethyl benzene oxidation under the same conditions at 70, at acetonitrile media, on catalyzer Со-PP are shown in Figure 8.
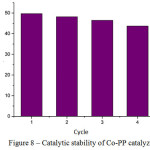 |
Figure 8: Catalytic stability of Со-PP catalyzer |
At the first cycle of reaction the conversion of ethyl benzene into oxygen-containing products to be 49,7 %. After the catalyzer to be derived from the reaction mass with further washed by water and drying at vacuum oven it to be placed back to the reactor for conducting the second cycle, with fresh portion of organic substrate and oxidizer. Thereat, the substrate conversion for 1.5% has lowered. At the next cycles the gradual lowering of catalyzer activity to be observed. At the tenth cycle the catalyzer activity has lowered for 50% comparing the initial activity. This is obviously associated with gradual decomposition of polyporphyrin metal complex under impact of hydrogen peroxide.
Conclusion
Thus, on the basis of conducted examinations there has been developed the low temperature heterogenetic polyporphyrin metal complexes for oxidation of alkylaromatic hydrocarbons by hydrogen peroxide under the soft conditions. Data comparison over the catalytic activity of all examined catalyzers has shown that the favorable conditions for conducting the reaction of hydrogen peroxide decomposition at the atmospheric pressure are the temperature of 70. The process of oxidation of cumene, toluene and ethyl benzene to be conducted under the temperature of 60-80 by hydrogen peroxide at the acetonitrile media and other solvents. The most active catalyzer is Co-PP.
References
- Matiyenko, L.I.; Mossolova, L.А.; Zaikov, G.Е. Chemical progress 2009 No. 3 (78) 228-247 (in Russian).
- Punniyamurthy ,T.; Subbarayan, V.; Iqbal, J.; Chem. Rev. 2005.105, 6 ,2329-2364.
- Zipplies, M.F.; Lee, W.A.; Bruice, T.C. J. Am. Chem. Soc.1986 108, 15, 4433-4445.
- Battioni, P.; Renaud, J.P.; Bartoli, J.F.; Reina-Artiles, M.; Fort, M.; Mansuy, D. J. Am. Chem. Soc. 1988 ,110., 8462-8471.
- McLain, J.L.; Lee, J.; Groves, J.T. Biomimetic Oxidations Catalyzed Ed. B.L. Meunier: Imperial College Press 2000., 91.
- Zhang, X.B.; Guo, C.C.; Xu, J.B.; Yu, R.Q. Journal of Molecular Catalysis A: Chem. 2000, 154 31-38.
- Gherassimova, О.А.; Shpakovskiy, D.B.; Milayeva, Е.R.; Loulowdi, М.; Hadjiliadas, Н. Moscow university Bulletin. Series 2. Chemistry 2007 48, 5. 322-328. (in Russian).
- Esmelindro, M.C.; Oestreicher, E.G.; Márquez-Alvarez, H.; Dariva, C.; Egues, S.M.S.; Fernandes, C.; Bortoluzzi, A.J.; Drago, V.; Antunes, O.A.C. Journal of Inorganic Biochemistry 2005 .,99 .,2054-2061.
- Solovieva, A.B.; React. Polym. 1991 16, 1,9-18.
- Jun, K.W.; Shim, E.K.; Park, S.E.; Lee, K.W. Bull. Korean Chem. Soc. 1995 16, 5, 398-407.
- Rahiman Kalilur, A.; Bharathi Shanmuga, K.; Sreedaran, S.; Narayanan, V. Catal Lett. 2009 127., 175-180.
- Vianna Rosa, I.L.; Manso, C.M.C.P.; Serra, O.A.; Iamamoto, Y. Journal of Molecular Catalysis A: Chemical 2000 .,160 ,199-208.
- Shulpina, L.S.; Takaki, К.; Strelkova, Т.V.; Shulpin, G.B. Oil chemistry 2008 48,.3., 220-223. (in Russian).
- Traylor, T.G. J.Am.Chem.Soc. 1991 113, 20 ,7821-7830.
- Wang, R.M.; Wang, Y.P. Chemistry Letters 1993, 5, 855.
- Alan, D. J. Am. Chem. Soc. 1966, 32 ,476.

This work is licensed under a Creative Commons Attribution 4.0 International License.









