Membrane and Adsorption Processes for Removing of Organics and Inorganics from Urban Wastewaters
Majlinda Daci-Ajvazi1*,Bashkim Thaçi1, Nexhat Daci2 and Salih Gashi2
1Chemistry Department, University of Prishtina, Republic of Kosovo.
2Academy of Sciences and Arts of Kosovo, Republic of Kosovo.
Corresponding Author: bthaqi75@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/320510
Since in Kosovo there are still no water purification plants and untreated wastewaters are discharged in environment, in this paper we’ve studied methods for removing of different organic and inorganic pollutants from Kosovo urban wastewaters. For best results we’ve used two methods, reverse osmosis and adsorption. For reverse osmosis, all samples were pretreated with coagulant (FeSO4) and flocculant (CaO) and then treated with reverse osmosis membranes. For adsorption, we used Kosovo coal ash and bentonite, both natural and low cost adsorbents. The analysis of experimental results shows that removing of organic and inorganic pollutants by reverse osmosis was very effective and removed from 93-98% of organics and almost 100% of heavy metal ions. Efficiency of coal ash in removing organics from natural waters was from 88-95%, while the efficiency of bentonite was 77-88%, while removing of heavy metal ions by coal ash was from 79-100% and by bentonite was from 50-92% respectively.
KEYWORDS:coal ash; reverse osmosis; membranes; bentonite; adsorbtion
Download this article as:| Copy the following to cite this article: Daci-Ajvazi M, Thaçi B, Daci N, Gashi S. Membrane and Adsorption Processes for Removing of Organics and Inorganics from Urban Wastewaters. Orient J Chem 2016;32(5). |
| Copy the following to cite this URL: Daci-Ajvazi M, Thaçi B, Daci N, Gashi S. Membrane and Adsorption Processes for Removing of Organics and Inorganics from Urban Wastewaters. Orient J Chem 2016;32(5). Available from: http://www.orientjchem.org/?p=21322 |
Introduction
Coal as fossil fuel is the most abundant fossil fuel resource in most countries including Kosovo, and many studies described it as the bridge to future energy systems, while stressing world coal prospects, environmental problems, resources and energy projections1-3. Many studies are also oriented on finding ways for improving the adsorption properties of coal through different modification4,5.
Kosovo, although small in area (10,908 km2), it is rich in energy resources and lignite type coal is the main national natural resource. Coal, as a lignite type, was and still is a very important natural resource that was extensively used as main energy resource for a half century. In this process, the existing thermal power plans discharge extremely large ash amounts, deposited in landfills which in turn largely pollute surface and underground waters and environment6,7.
Lignite type coal in Kosovo was extensively used in electricity power plants. Unfortunately the exploitation of lignite was done without respect to environmental capacity of the country and almost has created an environmental bomb for population. Uncontrolled burning of lignite in power plants each year left in Kosovo environment about 2 million tons of fly and bottom ashes, more than 100 thousand t of sulphur, of which more than 50% organic, over 11 t of arsenic, more than 2 t of beryllium, 1 t of cadmium, 351t of nickel, 492 t of titanium, 191 t of manganese, 0.48 t vanadium, etc. This uncontrolled burning of lignite in Kosovo power plants, jeopardize both our public health and our environment8,9.
Since coal is main natural resource of Kosovo, we’ve used it in reverse osmosis for modification of membranes and in adsorption for removing of different pollutants from natural and wastewaters. Since Kunst and Sourirajan10 produced productive asymmetric cellulose acetate membranes for a low pressure reverse osmosis application many investigations have been initiated for further development of these membranes. Exploitation of new membrane materials, modification of existing membrane materials by using chemical or physical methods such as plasma modification radiation induced grafting, surface chemistry reaction, surface coat and blend method have been frequently used. Especially, the blend method has become widespread and efficient to prepare a good membrane11. In order to obtain membranes with specific properties, additional additives can be added to the casting solution. Coal was used as new material to prepared heterogeneous reverse osmosis membranes12. It was found that aryldiazonium salts at low concentration in their organic or aqueous are materials able to be electrochemically reduced on carbon semiconductors13-15 or conductors16. Therefore, this coal was modified with 4- nitrobenzene diazonium salt in acetonitrile or hydrochloric acid. The membranes from cellulose acetate and coal modification are more productivity in comparison than that non modified coal17. The modified coal with 4- nitrobenzene diazonium salt shows encouraging results for preparation of heterogeneous reverse osmosis membranes.
Reverse osmosis membrane can be well protected by pretreatment, as it is a significant determinant for employing water reuse plants in remote areas18. Currently, reverse osmosis feed pretreatment is mainly used to reduce suspended and colloidal material in the feed water. Conventional pretreatment includes disinfection, flocculation/coagulation and filtration processes, including microfiltration or ultra filtration19. In the last decades, on many occasions, the membrane processes looks more and more promising as separation processes in industry and waste water treatment.
Among various water purification and recycling technologies, adsorption is a fast, inexpensive and universal method. Activated carbon is considered as universal adsorbent for the removal of diverse types of pollutants from water. The use of various low-cost adsorbents (from organic precursors like fertilizer industry wastes, fly ash, biomass, peat, fruit stones etc. and inorganic precursors including red mud, clays, blast zeolites etc.,) as substitutes for the costly activated carbon, have been extensively studied and have led to the rapid growth of research interests in this field20-22. Heavy metals pose a significant threat to environment and public health because of their toxicity, accumulation in the food chain and persistence in nature. Adsorption technology is one of the best water treatment technologies and has been used extensively for the uptake of toxic metal ions from ground, surface and wastewater.
Continuously increasing demand for raw materials and the limited availability of natural resources, as well as extensively used lignite type coal for producing energy gave rise to the investigation of possible reuse of fly ash and bentonite for extracting valuable trace elements or as adsorption material for heavy metals as pollutant in water streams23-26. Bentonite, another natural occurring material shows a wide range of industrial applications. It’s abundance in most countries and its low cost makes it a suitable adsorbent for the removal of many pollutants27. For removal of organics and inorganics from water samples we’ve used coal ash as adsorbent, which meet dual goals of disposal and treatment, and as comparison we’ve used bentonite. Although both used adsorbents are alum silicates, they differ in their composition. There is predominance of alkaline components (CaO and MgO) in Kosovo coal ash and in bentonite there is predominance of acid components (SiO2, Al2O3).
Materials and methods
Coal modification
The coal specimens were treated with boiling water (353K) under stirring conditions. The residual coal after filtering was dried at 378 K to constant weight. Typically 8 g of this coal was dispersed in 100 cm3 phosphoric acid containing 15 mM of N2C6H4BF4. The solution was stirred at temperature 278 K controlled water bath for 5h. The reaction mixture was filtered with a 589 Blue ribbon and then the modified coal was washed by successive aliquots of water. Finally the modified coal was dried at 378 K to the constant weight, ground and sieved. The coal fractions of sieve size of ~ 170 mesh were used in this study.
Film casting details
The film casting details for the investigated membranes are casting solution composition cellulose acetate (E 398-3) 10 wt%, modified coal with aryldiazonium salt 15 wt%, with corresponding amounts of acetone 61.3 wt%, water 12.25 wt%, and magnesium perchlorate 1.45 wt% at temperature of casting solution and casting atmosphere 297K and ambient air (relative humidity 60%). The films were cast on a clean glass plate (22×38 cm) with the use of a metal cylinder with uplifted edges to obtain the required film thickness (0.12mm). The glass plate was kept at the same temperature as the casting solution. The casting solution temperature and the external conditions of solvent evaporation time (0s) during film casting were the same for all series of films studied. The cast solution was immediately dipped into a gelatin bath consisting ice cold water (273K). The duration of the film setting in ice cold water was 1h. Before the reverse osmosis experiment, the membranes were preshrunk under water at different temperatures (the temperature of water bath was controlled to within ± 0.5K) and initially each film was subjected to pure water pressure treatment for 1h at 20% higher pressure than that to be used in reverse osmosis run. The surface area of the membranes investigated was 11.92 cm2.
Permeate flow rate, referring to the membrane-permeated solution corrected to 298K, and the rejection factor R, defined as:
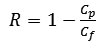
Cp and Cf – permeate and feed concentrations (g/dm3).
Pretreatment step
First the urban waste waters were treated by FeSO4 and CaO in proportion 0.05:0.125 g/dm3. This step was used as pretreatment method. After that these waste waters are treated with reverse osmosis membranes. After pretreatment the pH was increase till 10.7, so we used some drops H2SO4 to decrease pH to 7, because the membranes degrade in this pH.
Reverse osmosis apparatus
The membranes, five samples of each type, were tested in a laboratory reverse osmosis apparatus illustrated schematically in Fig. 1
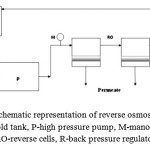 |
Figure 1: Schematic representation of reverse osmosis set-up. H–hold tank, P-high pressure pump, M-manometer, RO-reverse cells, R-back pressure regulator |
Adsorption
As adsorbents we used coal ash from Kosovo thermo power plants (Kosova A and Kosova B) and bentonite. They were dried at 105ºC and calcinated at 800°C. Physical-chemical characteristics of adsorbents were determined with standard analytical methods (Table 3).
1g of coal ash/bentonite was added to a certain volume of water sample (100 cm3). Solution was continuously mixed with magnetic stirrer for 30 minutes. After completion of the reaction time, the mixture was filtered and analyzed.
Physical-chemical analysis of the water samples: turbidity (TUR), conductivity (EC), pH, temperature, dissolved oxygen (DO), chemical oxygen demand (COD), biological oxygen demand (BOD), total organic carbon (TOC), total suspended solids (TSS), total dissolved solids (TDS), surfactants (SUR), nitrate ions (NO3–), phosphate ions (PO43-), total phosphorus (Ptot), ammonium ions (NH4+), sulphate ions (SO42-) and analysis of heavy metal ions: Pb2+, Cd2+, Fe2+, Ni2+ , Cr3+, Zn2+, Mn2+ and Cu2+ were done before and after treatment with FeSO4/CaO, reverse osmosis, coal ash and bentonite. Temperature, pH, EC and TDS were measured directly with HI98130 instrument. COD, BOD, TOC, SUR, NO3– were measured directly with Pastel UV- Secoman instrument. DO was measured directly with instrument HI9146. For PO43-, (ammonium molibdate method), total phosphorous Ptot (ammonium persulfate method) and for NH4+ Visible Light premiums Secoman spectrophotometer was used. For SO42- standard sodium thiosulfate titration method was used. Heavy metal ions were analyzed with atomic absorber 400 Perkin Elmer.
Samples: S1 – urban waste waters of capital city of Kosovo, Pristina, S2 – urban waste waters of Prizren city, S3 – urban waste waters of Mitrovica city and S4 – urban waste waters of Ferizaj city.
Results and discussion
Extensively used lignite type coal for producing energy, abundance of bentonite in nature and also their low cost makes them suitable adsorbents for the removal of many pollutants. In terms of physical-chemical properties, chemical composition of ash varies greatly, depending on the type of coal and its origin. Coal ash from our lignite type coal has specific inorganic composition. The results that were achieved in determining chemical composition of Kosovo coal ash and bentonite are shown in Table 1.
Table 1: Chemical composition of Kosovo coal ash and bentonite
| Adsorbents | Loss on ignition |
SiO2 |
Al2O3 |
Fe2O3 |
CaO |
MgO |
Na2O |
K2O |
TiO2 |
|
Coal ash % |
2.20 |
26.75 |
4.00 |
10.77 |
41.48 |
4.36 |
1.42 |
0.16 |
0.50 |
|
Bentonite % |
6.091 |
65.11 |
9.07 |
3.69 |
0.68 |
1.27 |
– |
– |
– |
From the features shown in Table 1, it is expected that metal ions in water samples, will mostly be absorbed by silicon oxides, aluminum oxides or iron oxides, or by influence of combination of these oxides. It is assumed that the predominance of alkaline components (CaO and MgO) in Kosovo coal ash, will also affect the precipitation of some metal ions.
Comparative analysis of the two materials used as natural adsorbents, show the differences in their composition. Although both used adsorbents are alumosilikates, bentonite differs from the domination of acidic constituents (80%) to those alkaline (20%), while in coal ash dominates alkaline constituents (80%) to those acidic (20%)
From the features shown in Table 2, we can see that these waters have pH 7.93-8.94 and high electrical conductivity and TDS. From data for COD, BOD and TOC it can be seen that these waters are polluted.
Experience from membrane waste water treatment has demonstrated that key design parameters must be followed to prevent rapid membrane fouling, and thus reduce height system maintenance costs and significant downtime. Chemical pretreatment process is one of the most popular methods for waste water pretreatment due to its low cost and simplicity. Pretreatment objectives, is to make the waste water to the reverse osmosis compatible with the membrane. Pretreatment is required to increase the efficiency and life expectancy of the membrane elements by minimizing fouling, scaling and degradation of the membrane. Inadequate pretreatment often necessitates frequent cleaning to restore product flux and rejection. This will result in excessive chemical cleaning costs, and in severe cases will result in permanent loss of performance, membrane degradation and therefore shorter membrane life. So, we used coagulant (FeSO4) and flocculant (CaO) to pretreated water samples (S1, S2, S3, and S4), see Table 2, 3 and Figure 1, 2.
From Table 2 and Figure 2 it can be seen the effect of chemical pretreatment with FeSO4/CaOin reduction of COD, Pt, NO3– by 53.4%, 58.9%, 60.1%, As we see from the data this step is very important. After the pretreatment samples were treated with reverse osmosis (RO) membranes. RO membranes can remove dissolved solids which can’t be removed by chemical and biological or other conventional municipal treatment process. Same tables and figures show the results of RO membranes. From these data we can see that RO membranes have shown significant reduction of: TDS (95-97%), COD (93-98%), BOD (96-97%), TOC (97%), EC (95-97%). The flux of these membranes was 4.9×10-2 m3/h/m2.
Table 2: Results from physical chemical analysisof water samples
|
Sample |
t ˚C | pH | ECμS/cm | TDS mg/dm3 | TSS mg/dm3 | DOmg/dm3 | CODmg/dm3 | BOD mg/dm3 | TOC mg/dm3 | SUR mg/dm3 | TUR NTU | NO3– mg/dm3 | Ptot mg/dm3 | NH4+ mg/dm3 | |
| S1 | Untr. |
18.2 |
7.93 |
798 |
438 |
238 |
5.5 |
133 |
63.0 |
43.6 |
7.0 |
22.1 |
6.4 |
5.16 |
2.96 |
| FeSO4/ CaO | 20 | 10.2 | 724 | 394 | 122 | ↑ | 62 | 31 | 25 | 1.4 | 3.22 | 2.5 | 2.12 | 1.38 | |
| RO | 20 | 7.3 | 33 | 14 | 5.1 | ↑ | 4.0 | 1.1 | 0.7 | 0.0 | 0.0 | 0.04 | 0.00 | 0.125 | |
| CA | 20.1 |
14 |
1564 |
782 |
32.5 | ↑ | 15.0 |
4.3 |
4.70 |
0.0 | 0.0 | 0.0 | 0.20 | 0.16 | |
| BE | 20.1 | 9.9 | 840 |
482 |
48 | ↑ | 24.0 | 9.1 |
7.6 |
1.1 |
0.0 | 0.0 |
0.59 |
0.22 |
|
| S2 | Untr. | 15.7 | 8.54 | 568 | 289 | 117 | 5.52 | 71.4 | 27 | 20 |
4.0 |
30.98 | 5.7 | 5.79 | 3.59 |
| FeSO4/ CaO | 21 | 10.5 | 517 | 261 | 56 | ↑ | 35 | 14 | 11 | 1.1 | 2.82 | 1.5 | 1.07 | 1.28 | |
| RO | 21 | 7.2 | 23 | 12 | 1.3 | ↑ | 0.9 | 0.4 | 0.3 | 0.3 | 0.0 | 0.01 | 0.00 | 0.05 | |
| CA | 21 |
>14 |
1632 |
818 |
32.0 | ↑ | 6.0 |
1.7 |
2.3 |
1.1 |
0.0 |
0.0 |
0.6 |
0.46 |
|
| BE | 21 | 10.8 | 623 |
308 |
36.8 | ↑ | 12 | 4.5 |
4.7 |
1.6 |
0.0 | 0.3 |
1.2 |
1.04 |
|
| S3 | Untr. |
19.3 |
8.94 |
722 |
361 |
65 |
5.65 |
84.6 |
39.6 |
30.6 |
0.8 |
9.3 |
5.0 |
3.42 | 0.51 |
| FeSO4/ CaO | 21 | 10.7 | 658 | 328 | 32 | ↑ | 44 | 19 | 13 | 0.2 | 1.81 | 1.6 | 0.88 | 0.28 | |
| RO | 21 | 7.4 | 18 | 10 | 1.1 | ↑ | 0.8 | 0.6 | 0.4 | 0.0 | 0.0 | 0.01 | 0.00 | 0.01 | |
| CA |
21 |
>14 |
2030 |
810 |
0.0 |
↑ |
4.8 |
1.8 |
4.8 |
0.0 |
0.0 |
0.0 |
0.3 |
0.04 |
|
| BE |
21 |
9.44 |
834 |
392 |
25.2 |
↑ |
10.2 |
8.2 |
5.6 |
0.2 |
0.0 |
0.1 |
0.6 |
0.21 |
|
| S4 | Untr. |
17.5 |
8.35 |
780 |
382 |
122 |
6.8 |
87 |
38.4 |
26.8 |
0.6 |
26.06 |
3.0 |
4.45 |
2.73 |
| FeSO4/ CaO | 22 | 10.4 | 716 | 339 | 73 | ↑ | 41.5 | 17.8 | 13.1 | 0.15 | 1.98 | 1.20 | 0.11 | 1.003 | |
| RO | 22 | 7.6 | 29 | 19 | 2.0 | ↑ | 0.8 | 0.5 | 0.3 | 0.00 | 0.00 | 0.02 | 0.00 | 0.074 | |
| CA |
20.1 |
>14 |
1175 |
587 |
27.8 |
↑ |
7.8 |
3.8 |
2.4 |
0.0 |
0.0 |
0.0 |
0.6 |
0.13 |
|
| BE |
20.1 |
9.8 |
820 |
412 |
30.5 |
↑ |
13.2 |
7.0 |
4.6 |
0.1 |
0.0 |
0.0 |
1.05 |
0.71 |
|
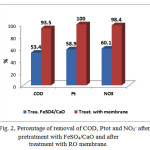 |
Figure 2: Percentage of removal of COD, Ptot and NO3– after pretratment withFeSO4/CaO and after treatment with RO membrane |
Unt – untreated sample; RO – reverse osmosis; CA – coal ash; BE – bentonite; ↑ – after treatment DO was higher than instruments detection limit.
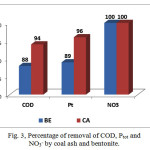 |
Figure 3: Percentage of removal of COD, Ptot and NO3– by coal ash and bentonite. |
From Table 2 and Figure 3 data results for DO 5.5-6.8 mg/dm3, COD 71.4-133 mg/dm3, BOD 27-63 mg/dm3, and TOC 20-43.6 mg/dm3 show that we are dealing with polluted areas which correlate with TDS 289-438 mg/dm3 and TSS 65-238 mg/dm3 results.
Reduction of TSS plays a significant role in modern wastewater treatment, since the SS serves as an adsorbent for heavy metals and polychlorinated biphenyls (PCBs). Results from Table 2 show that removal of TSS by coal ash was very effective from 73-100% and by bentonite from 61-80%. The presence of excess nitrogen and its compounds has a negative impact on the environment. Nitrogenous compounds play an important role in water pollution, therefore, the control of them in water has vital importance. Efficiency of removal of nitrate ion and ammonium ion from the treated water samples was high. Removal of nitrate ion by coal ash was 100%, with bentonite 95-100%, while removal of ammonium ion by coal ash was 87-95% and by bentonite 71-93% respectively. High levels of organic pollutants into river water affect the suitability of river water for human use and it stimulates the growth of algae and aquatic plants. Various treatment methodologies have been used for the removal of organic pollutants but most of them are complicated and time-consuming, except for adsorption methods, which are low cost and easy to use. Coal ash and bentonite were very effective adsorbents for removal of organics. Removalof COD by coal ash was from 83-94% while with bentonite was 73-88%. The removal of total phosphorus by adsorption is simple and convenient. Results from Figure 2 show the removal of total phosphorus by coal ash was from 87-96% while with bentonite was 76-89%.
Table 2 shows that pH before and after treatment with coal ash are very different. pH values revolve primarily in line with the guiding values for waters discharged into natural ecosystems, before treatment they range from 7.93-8.94, after treatment with coal ash the pH values were increased in 14, which is expected due to the composition of the ash, and after treatment with bentonite the pH values were increased from 9.44 – 10.8 respectively. As for conductivity values before treatment they vary from 568-798 µS/cm and after treatment with coal ash the EC values were increased from 1175-2030 µS/cm, while after treatment with bentonite EC values were 623-840 µS/cm.
Table 3: Analasys of metal ions before and after treatment with coal ash and bentonite and their precentage of removal
|
Sample |
Fe2+mg/dm3 |
% |
Ni2+mg/dm3 |
% |
Cr3+mg/dm3 |
% |
Zn2+mg/dm3 |
% |
Mn2+mg/dm3 |
% |
Cu2+mg/dm3 |
% | Pb2+mg/dm3 |
% |
Cd2+mg/dm3 |
% |
|
| S1 | UT |
0.073 |
0.780 |
0.290 |
0.102 |
0.170 |
0.302 |
0.047 |
0.035 |
||||||||
| FeSO4/CaO | 0.031 | 59.5 | 0.028 | 64.1 | 0.110 | 62 | 0.042 | 58.8 | 0.071 | 58.2 | 0.110 | 63.5 | 0.018 | 61.7 | 0.016 | 51.4 | |
| RO | 0.002 | 93.5 | 0.001 | 96.4 | 0.004 | 96.3 | 0.001 | 97.6 | 0.001 | 98.6 | 0.004 | 96.4 | 0.001 | 94.4 | 0.001 | 93.75 | |
| CA |
0.00 |
100 |
0.07 |
91 |
0.04 |
86 |
0.03 |
90 |
0.00 |
100 |
0.02 |
93 | 0.01 |
79 |
0.00 |
100 |
|
| BE |
0.013 |
82 |
0.19 |
76 |
0.09 |
69 |
0.06 |
80 |
0.03 |
82 |
0.10 |
67 | 0.019 |
60 |
0.01 |
71 |
|
| S2 | UT |
0.068 |
0.038 |
0.055 |
0.014 |
0.12 |
0.16 |
0.019 |
0.003 |
||||||||
| FeSO4/CaO | 0.032 | 52.5 | 0.014 | 63.1 | 0.022 | 60 | 0.006 | 57.1 | 0.05 | 58.3 | 0.071 | 55.6 | 0.007 | 63.1 | 0.002 | 33.3 | |
| RO | 0.003 | 90.6 | 0.002 | 85.7 | 0.003 | 86.3 | 0.000 | 100 | 0.003 | 94 | 0.002 | 97.1 | 0.000 | 100 | 0.000 | 100 | |
| CA |
0.01 |
85 |
0.001 |
97 |
– |
100 |
0.001 |
93 |
0.003 |
97 |
0.005 |
97 |
– |
100 |
– |
100 |
|
| BE |
0.02 |
71 |
0.004 |
89 |
0.006 |
89 |
0.005 |
64 |
0.02 |
83 |
0.05 |
69 |
0.004 |
79 |
0.001 |
67 |
|
| S3 | UT |
0.071 |
0.104 |
0.010 |
0.219 |
0.104 |
0.11 |
0.239 |
0.032 |
||||||||
| FeSO4/CaO | 0.030 | 57.7 | 0.048 | 53.8 | 0.006 | 40 | 0.091 | 58.4 | 0.048 | 53.8 | 0.051 | 53.6 | 0.118 | 50.6 | 0.015 | 53.1 | |
| RO | 0.000 | 100 | 0.002 | 95.8 | 0.001 | 83.33 | 0.002 | 97.8 | 0.001 | 97.9 | 0.002 | 96 | 0.004 | 96.6 | 0.002 | 86.6 | |
| CA |
0.08 |
89 |
0.01 |
90 |
– |
100 |
0.003 |
98 |
– |
100 |
0.002 |
98 |
0.01 |
95 |
0.001 |
96 |
|
| BE |
0.13 |
82 |
0.03 |
71 |
0.002 |
80 |
0.028 |
87 |
0.04 |
62 |
0.008 |
92 |
0.05 |
79 |
0.007 |
78 |
|
| S4 | UT |
0.079 |
0.069 |
0.033 |
0.002 |
0.076 |
0.006 |
0.006 |
0.017 |
||||||||
| FeSO4/CaO | 0.040 | 49.4 | 0.021 | 69.5 | 0.013 | 60.6 | 0.001 | 50 | 0.031 | 59.2 | 0.003 | 50 | 0.002 | 66.6 | 0.007 | 58.8 | |
| RO | 0.00 | 100 | 0.001 | 95.2 | 0.001 | 92.3 | 0.000 | 100 | 0.001 | 96.7 | 0.000 | 100 | 0.000 | 100 | 0.002 | 71.4 | |
| CA |
– |
100 |
0.003 |
95 |
0.001 |
97 |
– |
100 |
– |
100 |
– |
100 |
– |
100 |
0.002 |
88 |
|
| BE |
0.007 |
91 |
0.01 |
85 |
0.01 |
70 |
0.001 |
50 |
0.024 |
68 |
0.002 |
66 |
0.002 |
67 |
0.006 |
65 |
|
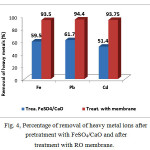 |
Figure 4: Percentage of removal of heavy metal ions after pretratment withFeSO4/CaO and after treatment with RO membrane |
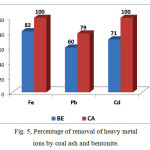 |
Figure 5: Percentage of removal of heavy metal ionsby coal ash and bentonite. |
From the features shown in Table 3 and Figure 4 it can be seen the effect of chemical retreatment with FeSO4/CaOin removal of heavy metal ions Fe2+, Pb2+ and Cd2+ by 57.5%, 61.7%, 71.4%. Same tables and figures show the results of RO membranes. From these data we can see that RO membranes have shown almost complete removal of heavy metals: Fe2+ (90-100%), Ni2+ (85-96%), Cr3+ (83-96%), Zn2+ (97–100%), Mn2+ (94-98%), Cu2+ (96-100%), Pb2+ (94-100%) and Cd2+ (71-100%). These data indicates that resulting membranes are readily available and effective for treatment of municipal waste waters. The development of these membranes is of great interest for wide variety of low pressure reverse osmosis membranes especially for waste water treatment.
From Table 3 and Figure 5 it can be seen that both used absorbent showed very good sorption properties. Percentage of removal of heavy metal ions with coal ash was from 85-100% while with bentonite was from 50-92%. Increasing pH during adsorption indicates that the high efficiency of Kosovo coal ash in removing of metal ions can be attributed to two processes, the adsorption and precipitation process as a result of hydrolysis of the alkaline oxides in sodium and magnesium hydroxides which help precipitation of certain metal ions. The adsorption process is a result of characteristic structure of our coal, its high porosity and large surface area.
Conclusion
Based on the results that were achieved we can conclude that success of membrane processes depends on proper pretreatment, chemical control and RO membranes that are resistant to fouling. Data from this paper shows that these membranes are readily available and effective for treatment of municipal waste waters. The development of these membranes is of great interest for wide variety of low pressure reverse osmosis membranes especially for waste water treatment. Kosovo coal ash and bentonite show good sorption capacity toward heavy metal ions and other pollutants with high sorption rates of over 90% of the total adsorption which can be obtained in thirty minutes of contact time.
References
- Ishaq, M., Saeed, K. Ahmad, I. Sultan, S., Coal Ash as a Low Cost Adsorbent for the Removal of Xylenol Orange from Aqueous Solution, Iranian Journal of Chemistry and Chemical Engineering, 2014,. 33, 1.
- Geetha V. Ram, Vaishali, S. A comparative study on the removal of heavy metals by adsorption using fly ash and sludge: A review, International Journal of Application or Innovation in Engineering & Management, 2013,. 2(7), 2319 – 4847.
- WorldEnergy: Looking ahead to 2020; Published for the World Energy Conference by IPC Science and Technology Press, London.and cadmium concentration and correlation with biochemical parameters in blood of human populationnearby Kosovo thermo power plants,American Journal of Biochemistry and Biotechnology,2008, 4 (3): 273-276
- Samson, B. Sanja, V.,Evaluation and Treatment of Coal Fly Ash for Adsorption Application, Leonardo Electronic Journal of Practices and Technologies, ., 2008, 12., 37-48, , ISSN 1583-1078.
- Wang, S. Soudi, M. Li, L. Zhu, ZH., Coal ash conversion into effective adsorbents for removal of heavy metals and dyes from wastewater, Journal of Hazard Materials, 2006, 243-51
CrossRef - Daci, N.M., , Environmental Protection Engineering,1989, 4, 3-4.
- Daci, N.M., Inovation, Industried Progress and Environment, 1991, 1-9, Strasburgh.
- Zeneli, L., Daci, N., Daci-Ajvazi, M., Paçarizi, H., Effects of pollution on lead
- Zeneli, L., Daci, N., Paçarizi, H., Daci-Ajvazi, M., Interaction between cadmium and calcium in human blood at smokers, American Journal of Pharmacology and Toxicology, 2010, 5(1): 48-51
CrossRef - Kunst, B., Sourirajan, S., Development and performance of some porous cellulose acetate membranes for reverse osmosis desalination, J. Appl. Polym. Sci., 1970, 14, 2559.
CrossRef - Zhou, Ch., Wang, Zh., Liang, Y., Yao, J., Study on the control of pore sizes of membranes using chemical methods Part II. Optimization factors for preparation of membranes, Desalination, 2008, 225, 123-138
CrossRef - Gashi, S.T., Daci, N.M, Selimi, T., Reverse Osmosis Properties of Cellulose Acetate-Coal membranes. Environmental. Menagment for Developing Countries, 1984, 405, 1-9.
- Allongue P., Delamar M., Desbat B., Fagebaume O., Hitmi R., Pinson J. and Saveant J. M.), Covalent modification of carbon surfaces by aryl radicals generated from the electrochemical reduction of diazonium salts, Journal of American Chemical Society., 1996, 119, 201-207Chemical Abstract, 1996, 125, 144-212.
- Belmont J.A., Amici R.M., Galloway C.P., (Cabot Corp.) PCT WO 18688,
- Toupin M, and Belanger D, Thermal stability study of aryl modified carbon black by in situ g enerated diazonium salt, Journal of Physical Chemistry, 2007, 14, 5394-5401,
- Pinson J and Podvorica F., Attachment of organic layer to conductive or semiconductive surfaces by reduction of diazonium salts, Chemical Society Reviews. 2005, 34, 5, 429-439
CrossRef - Gashi S.T., DaciN.M., Podvorica F., Selim. T., Thaçi B., Effect of the modification time of coal with aryldiazonium salts on performance of cellulose acetate- coal heterogeneous reverse osmosis membranes, Desalination, 2009 240, 1-8
CrossRef - Zhang J, Northcott, K, Duke, M, Scales, P., Stephen, R.G., Influence of pretreatment combinations on RO membrane fouling, Desalination,2016., 393, 120-126,
CrossRef - Ebrahim S., Abdel-Jawad M., Bou-Hamad S., Safar M. Fifteen years of R & amp; D program in seawater desalination at KISR part I. pretreatment technologies for ROsystems, Desalination, 2001, 135, 141–153
CrossRef - Alinnor, I .J., Adsorption of heavy metal ions from aqueous solution by fly ash, Fuel, 2007, 86,. 853–857,
CrossRef - Gupta, V.K., Ali, I., Water Treatment for Inorganic Pollutants by Adsorption Technology, Environmental Water, 2013, 29-91,
CrossRef - Jiuhui, Q.U., Research progress of novel adsorption processes in water purification: a review, Journal of environmental sciences, 2008, . 20, 1-13,
CrossRef - Ahsan, S., Kaneco, S., Ohta, K., Mizono, T., Kani, K., , Use of some natural and waste materials for waste water treatment. Water Resorces. 2001.,35, 3738–3742,
- Gupta, V.K., Ali, I., Advances in water treatment by adsorption technology, Nature protocols, 2006, 1, 2661-2667
- Amit, B., Minocha, A.K.,, Conventional and nonconventional adsorbents for removal of pollutants. Indian Journal of Chemical Technology,2006., 13, 203-217,VAN 14.139.47.15.
- Kirk, D.W., Charles, Q., Jia, J.Y., Alan, L.T., Wastewater remediation using coal ash, Journal of Mateial Cycles and Waste Managment, 2003, 5, 5–8.
CrossRef - Vega, J.L., (2005), Bentonite as adsorbent of heavy metal ions from mine waste leachates, 9th International Mine waste Congress.

This work is licensed under a Creative Commons Attribution 4.0 International License.









