Dry Reforming of Methane Over Various Doping Level of Ce on La1-Xcexni0.4Fe0.6O3perovskite nano catalyst
Parastoo Dezvareh1, Moayad Hossaini Sadr2*, Hamidreza Aghabozorg3 and Karim Zare4
1Department of Chemistry, Science and Research branch, Islamic Azad University, Tehran, Iran.
2Departmentof Chemistry, Shahid Madani University of Azarbaijan, Tabriz, Iran.
3Research Institute of Petroleum Industry, Tehran, Iran.
4Department of Pure Chemistry, Shahid Beheshti University, Tehran, Iran.
Corresponding Author E-mail: sadr@azaruniv.edu
DOI : http://dx.doi.org/10.13005/ojc/320526
The La1-xCexNi0.4Fe0.6O3 (x= 0.1, 0.2 and 0.3) perovskite nanostructures were prepared via citrate sol-gel method. Synthesized samples were characterized by X-ray diffraction (XRD), temperature programmed reduction (TPR), and inductively coupled plasma (ICP) techniques. Specific surface area was determined by BET measurement. Scanning and transmission electron microscopy techniques were applied to study the morphology of the prepared samples. XRD patterns confirmed that a well-crystallized perovskite structure was formed in doping level up to x=0.2. Morphology results showed that homogenous particles in the range of nanometers were obtained through the applied synthesis method. TPR analysis revealed that by increasing the doping level of Ce up to 0.2 inthe preparedsamples, reduction process shifted to lower temperatures.The addition of Ce to La1-xCexNi0.4Fe0.6O3enhances the catalytic activity up to x= 0.2,but decreased significantly when x> 0.2. Catalytic activityof La1-xCexNi0.4Fe0.6O3perovskites in dry reforming of methane (DRM) were: LaNiO3>La0.8Ce0.2Ni0.4Fe0.6O3>La0.9Ce0.1Ni0.4Fe0.6O3> LaNi0.4Fe0.6O3>La0.7Ce0.3Ni0.4Fe0.6O3
KEYWORDS:Ce-substitution; perovskite; nanocatalyst; citrate sol-gel method; dry reforming of methane (DRM)
Download this article as:| Copy the following to cite this article: Dezvareh P, Sadr M. H, Aghabozorg H, Zare K. Dry Reforming of Methane Over Various Doping Level of Ce on La1-Xcexni0.4Fe0.6o3perovskitenanocatalyst. Orient J Chem 2016;32(5). |
| Copy the following to cite this URL: Dezvareh P, Sadr M. H, Aghabozorg H, Zare K. Dry Reforming of Methane Over Various Doping Level of Ce on La1-Xcexni0.4Fe0.6o3perovskitenanocatalyst. Orient J Chem 2016;32(5). Available from: http://www.orientjchem.org/?p=21829 |
Introduction
Dry reforming of methane (DRM)
CH4+ CO2 ↔ 2CO + 2H2ΔH298k = 247 kJ/mol (1)
CO2 + H2 ↔ H2O + CO ΔH298k = 41 kJ/mol (2)
has attracted academic and industrial researches to produce synthesis gas (syn-gas: H2+CO). This process converts two of the greenhouse gases including CH4 and CO2 to obtain syn-gas with an appropriate H2/CO ratio for several applications. It also reduces CO2 and CH4 emissions contributing to the greenhouse effect [1-2]. Synthesis gas can be applied in the Fischer–Tropsch synthesis industry to produce valuable chemicals [3,4]. The low H2/CO ratio is preferentially used for further applications such as methanol and liquid fuel synthesis [5, 6]. In recent years complex mixed metal oxides with the perovskite-type structure have been studied in catalysis and have been proposed for methane-reforming reactions in replacement of classical catalysts such as noble metal–based catalysts [7, 8]. Considerable researches have been focused on the activity and stability of perovskite-type oxides applied in this process [9, 10]. Perovskite-type oxides with general formula ABO3 (A: lanthanides, alkali metals, and alkali earth metals; B: transition elements) show promising performance as catalysts in dry reforming of methane. A is a large cation responsible for the thermal resistance and B is a redox cation responsible for catalytic activity [11].Perovskite-type oxides have many advantages such as high metallic dispersion, coke formation resistance and nanometer scale particles [12]. Possibility of total or partial substitution A- and/or B-sites cations leads to modifying their oxidation state, oxygen mobility in crystal lattice and the redox properties [13, 14]. Some examples of La substitution in La1-xMxNiO3 structure that leads to promoting catalytic activity are M= Ce [15,16,17] and Sr [18]. The resulting compounds show high resistance to carbon deposition because of the existence of Ni crystallite size and a large number of oxygen vacancies. Ce- substituted catalysts promote catalytic performance because of their high oxygen storage capacity and high lattice oxygen mobility [16]. Sutthiumporn et al. reported that substitution at B-site significantly improves structural stability and catalytic behavior [18]. Jahangiri et al. reported the performance of perovskite-type oxides La1–xSmxNiO3, LaNi1–xFexO3 and LaNi1-xCoxO3as catalyst precursors in combined reforming of methane (CRM) with CO2 and O2 by changing doping level (x) [19-21]. The properties of theperovskites greatly depends on the choice of A and B cations so we decided to study the effect of these changes. In the present research La1-xCexNi0.4Fe0.6O3perovskites with different doping level up to x = 0.3 were synthesized. The catalytic activity of the synthesized samples is investigated in dry reforming of methane.
Experimental
Preparation of Catalysts
La1-xCexNi0.4Fe0.6O3 samples have been prepared according to the citrate sol-gel method. Stoichiometric amounts of the cation at A site were used. La(NO3)3.6H2O(Merck, >99/9%), Ce(NO3)3.6H2O (Merck, >99%), Ni(NO3)2.6H2O (Merck, >99%), Fe (NO3)2.6H2O (Merck, >99%), citric acid (Merck, 99/5%), and ethylene glycol (99%) were applied in this method. Appropriate stoichiometric amounts of lanthanum, cerium, nickel and iron nitrates solution (1M) were mixed and stirred for 40 minutesat 80˚C, and then citric acid and ethylene glycol were added to thissolution with molar ratio of 1. The sol formation started and then the excess water was slowly removed within 8 h at 80˚C until transformation of sol into spongy gel occured and an amorphous gel was formed. The gel was finally dried at 110˚C for 24 h and calcined in air at 800˚C within 2 h. The heating rate was 1˚C/ min. up to 350˚C and 3˚C/ min. up to 800˚C.
Characterization Techniques
The specific surface areas of the samples were measured using N2 at 77 K on a Tristar 3000, Micrometrics apparatus by applying the multipoint BET method.Powder X-ray diffraction were obtained on a Phillips PW 1840 diffractometer equipment with a copper anode (CuKα monochromatized radiation source, λ=1.54056Å) to confirm the formation of the perovskite structure, phase purity and particle size determination. XRD profiles were collected in the 2Ө range, 10–80°, in steps of 5°/min. The catalyst phases were identified by comparing the observed results with the JCPDS database. The morphologies and determination of chemical compositions of the calcined catalysts were determined by scanning electron microscopy (SEM) images using a Philips XL30 microscope. Temperature-programmed reduction (TPR) was performed with a semiautomatic micrometrics TPD/TPR 29000 apparatus to investigate the reduction properties of the catalysts. Inductively coupled plasma (ICP) emission spectroscopy (Perkin-Elmer ICP/5500) was used to determine the metals.
Measuring Catalytic Activity
Catalytic activity in dry reforming of methane was evaluated as a function of the composition of the precursors and temperature of the reaction. The experimental tests of the catalyst activity were carried out using the feed gases (CH4, CO2, N2 and H2) with ultra-high pure grade (>99.999%) in micro-reactor by mass flow controllers (Model 5850, Brooks Instrument). The temperature of micro-reactor was measured and controlled with two thermocouples (Ni–Cr, K-Type, 0.5 mm diameter) and two PID thermo-controllers (Model Jumo iTRON08). The catalyst (0.4 g for all cases) was loaded in the middle of the reactor, and the feed gases at a total flow rate of 100 ml/min (WHSV=15 l/(h.g), CH4/CO2/= 1/1) under atmospheric pressure wereintroduced into the reactor as the default. Catalytic activity was studied under a temperature treatment between 600°C and 800°C. The reactants and products were analyzed by a gas chromatograph (Model 6890N, Agilent Technologies) provided with two detectors (FID and TCD). Prior to activity measurements, catalyst precursors were reduced in situ in a flow of 20% H2/N2 mixture (total flow rate of 50 ml/min) at 700°C for 2 h to generate the metal phase. In all tests, the performances were evaluated by conversions. The CH4 and CO2conversions, H2 and CO yields and H2/COratios are defined as follows[22]:
Results and Discussions
Characterization of the La1-xCexNi0.4Fe0.6O3samples
Crystalline Structure
The XRD patterns of the La1-xCexNi0.4Fe0.6O3samples forx = 0.0 up to 0.3 are displayed in Fig.1. For La1-xCexNi0.4Fe0.6O3samples( x = 0.0, 0.1, 0.2), diffraction lines are characteristic of the LaNiO3 perovskite phase (JCPDS No.: 88-633)at the absence of anyother crystalline phases. For substitution degree of x= 0.3 detailed examination of this pattern also revealed not only diffraction lines of the perovskite, but also lines indicating that Ceand Fe existed separately in the forms of CeO2 and Fe2O3. Lines representing NiO were not observed in this study, probably in the form of an amorphous phase[23].
 |
Figure 1: XRD patterns of La1-xCexNi0.4Fe0.6O3nanocatalysts.
|
Chemical Analysis and Surface Area Measurement
The chemical composition (wt.%) and surface area for some prepared samples are reported in Table 1. The ICP values reveal that the experimental data for La, Ce, Ni, Fe (wt.%) are close to the nominal value (reported in parenthesis). These results confirm the effectiveness of preparation procedure. BET surface areas of the catalysts are in the range of 5-22 m2g-1. Long exposure to high temperatures has led to low surface area solids. The crystallite size of the prepared samples was calculated byScherrer equation using the most intense peak, presented in Table 1. The results showed that the particle size of the prepared samples was in nanoscale.
Table 1: Elemental analysis by ICP (nominal values in parenthesis), BET surface areas, and crystallite size of some La1-xCexNi0.4Fe0.6O3perovskites (calculated by Scherrer equation).
|
La1-xCexNi0.4Fe0.6O3
|
||||||
|
X |
La(wt.%)* |
Ce(wt%) |
Ni(wt%) |
Fe(wt%) |
SA(m2/g) |
D (nm) |
|
0 |
69.8(70.9) |
00.0(00.0) |
12.2(12.0) |
18.0(17.1) |
5.4 |
49 |
|
0.1 |
64.1(63.7) |
6.5(7.0) |
12.3(12.0) |
17.1(17.3) |
13.4 |
43 |
|
0.2 |
55.4 (56.6) |
15.0(14.3) |
11.6(12.0) |
18.0(17.1) |
21.7 |
38 |
|
nominal values in parentheses* SA= BET surface area D= crystallite size |
||||||
Morphology
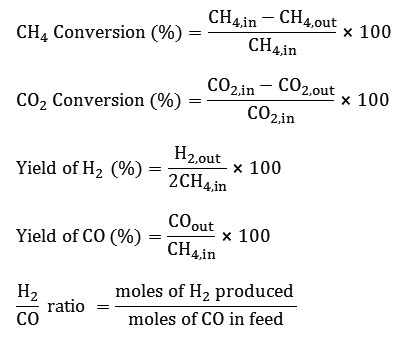
The morphology of the perovskites was studied by SEM and TEM. The SEM and TEM images are shown in Figs. 2 and 3, respectively. The SEM and TEM images show a uniform nanostructured texture with spherical particles, agglomerated and fine with a variable size of 40-50 nm.
 |
Figure 2: SEM images of La1-xCexNi0.4Fe0.6O3 ((x = 0.1) (A), (x = 0.2) (B), (x = 0.3) (C)). |
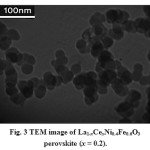 |
Figure 3: TEM image of La1-xCexNi0.4Fe0.6O3perovskite (x = 0.2). |
Reducibility Study of La1-xCexNi0.4Fe0.6O3
Since active sites for the reforming reaction are metalic nickel species, the catalysts must be reduced prior to application in the reaction tests. Fig. 4 indicates the results of TPR experiments. In the case of x = 0, the reduction of Ni3+ and Ni2+ ions represent weak and broad peaks [23,24], in the range of 300 and 550 ͦC, indicating that the Ni ions in the perovskite structure were not easily reduced. A similar result was observed when Ce was added x = 0.1. When the amount of added Ce increased to x = 0.2, the broad peaks were replaced with an intense peak, centered at 500 ͦC. This result shows that Ni ions were more easily reduced when x = 0.2.
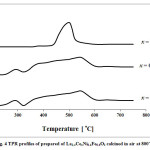 |
Figure 4: TPR profiles of prepared of La1xCexNi0.4 Fe0.6O3 calcined in air at 800˚C. |
Catalytic Activity
Catalytic performance of the prepared samples in the temperature range of 600-800˚C has been studied in DRM process.The CH4& CO2 conversions and H2 & COyields in the presence of La1-xCexNi0.4Fe0.6O3in different temperatures are presented in Fig. 5. As it is shown, CH4 and CO2 conversions increase by increasing the temperature and the CO2 conversions are always higher than that of CH4. This behavior can be due to consuming of CO2 in reaction equations 1 and 2. In addition, both H2 and CO yields increase by rising reaction temperature and the CO yields are higher than that of H2 in the presence of the La1-xCexNi0.4Fe0.6O3catalysts. This behavior is more significant at higher temperatures. In the presence of La1-xCexNi0.4Fe0.6O3sampleswith partial doping level of x = 0.3, there is no significant CH4 and CO2 conversions and product yields for each temperature. Fig. 6 indicates the CH4& CO2 conversions, and H2& CO yields versus time at 750ͦC temperature for La1-xCexNi0.4Fe0.6O3samples. These diagrams show that the CH4& CO2 conversions and H2& CO yields in the presence of the pure perovskite are higher than the catalysts containing more than one phase. Catalytic activity study of the prepared samples in fig. 7 also indicates that H2/CO ratio in the presence of La1-xCexNi0.4Fe0.6O3catalyst with doping level of x= 0.1 and x= 0.2 is ~1. It can be concluded that the reaction equation 1 is the main occurring reaction. H2/CO ratio is less than 1 in the presence of the catalyst withupper Ce doping level (x >0.2) containing more than one phase. This ratio is also less than 1 for the sample without Ce (LaNi0.4Fe0.6O3). In general, in the presence of La1-xCexNi0.4Fe0.6O3samples, CH4 and CO2 conversions in DRM process depended on the content of Ce. Thermal stability, high ionic conductivity, redox properties, and the oxygen transport properties of Ce played an important role in a good catalytic performance of these nanocatalysts[3]. High redox chemistry of cerium promotes the overall performance of the Ni-based DRM catalysts [14].
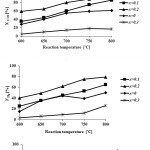 |
Figure 5: CH4 and CO2 conversions, and H2 and CO yields as a function of the reaction temperatureforreduced La1-xCexNi0.4Fe0.6O3samples in DRM process (CH4/CO2= 1/1 and WHSV=15 l/(h.g)). |
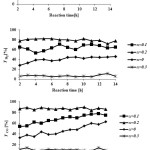 |
Figure 6: CH4 and CO2 conversions, H2 and CO yields as a function of the reaction time for reducedLa1-xCexNi0.4Fe0.6O3samples at 750˚C in DRM process (CH4/CO2= 1/1 WHSV=15 l/(h.g)). |
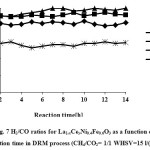 |
Figure 7: H2/CO ratios for La1-xCexNi0.4Fe0.6O3 as a function of the reaction time in DRM process (CH4/CO2= 1/1 WHSV=15 l/(h.g)). |
Conclusions
La1-xCexNi0.4Fe0.6O3( x = 0.1,0.2 ) perovskite nanocatalysts were prepared by citrate sol-gel method and evaluated as catalyst precursors in the dry reforming of methane. Spherical particles with crystalline size in nanometer scale and well-defined structure were obtained.
TPR analysis revealed that partial substitution of La by Ce in La1-xCexNi0.4Fe0.6O3perovskite structure generated a change in the temperature of nickel reducibility and reduction process shifted to lower temperatures.
Ce-substitution rises CH4& CO2 conversions and H2& CO yields by increasing both the BET surface area and the metal dispersion.
CH4 and CO2 conversions and H2& CO yields increased by increasing the temperature in the presence of the pure perovskite catalysts.
The most promising catalyst was the partially doped perovskite La0.8Ce0.2Ni0.4Fe0.6O3that performed highest catalytic activity in comparison with other doping levels of Ce.
The most important aim of this research is to investigate the appropriate H2/CO ratios in dry reforming of methane over La1-xCexNi0.4Fe0.6O3( x = 0.1, 0.2 ) perovskite nanocatalysts to obtain more applicable chemicals of syn-gas in future as a novel research. Syn-gas can be applied in the Fischer–Tropsch synthesis industry to produce valuable chemicals.
References
- Valderrama, G.;Kiennemann, A.;Goldwasser, M.R. J. Power Sources. 2010,195(7), 1765-1771.
- Khalesi, A.; Arandiyan, H.R.;Parvari, M. Chin. J. Catal. 2008, 29 (10),960-968.
- Valderrmaa, G.; Goldwasser, MR.; Navarro, CU.; Tatibouët, JM.; Barrault, J.; Dupeyrat, CB.; Martinez, F. Catal Today. 2005,107–108, 785–791.
- Ferreira-Aparicio, P.; Guerrero-Ruiz, A.; Rodriguez-Ramos, I. Appl. Catal. Gen.1998,170 (1), 177-187.
- Corthals, S.; Van Nederkassel, J.;Geboers, J.; De Winne, H.; Van Noyen, J.;Moens, B. Catal. Today.2008, 138, 28–32.
- Eltejaei, H.;Bozorgzadeh, HR.;Towfighi, J.;Omidkhah, MR.;Azari, M.;Zanganeh, R. Int. J. Hydrogen Energy, 2012, 37, 4107-18.
- Pereniguez, R.; Gonzalez-Delacruz, VM.;Holgado, J P.; Caballero, A. Appl. Catal. B.2010, 93(33), 346-353.
- Lima, SM.; Assaf, JM.;Peňa, MA.;Fierro, JL. Appl. Catal.A.2006,311, 94–104.
- OsojnikČrnivec, IG.;Djinović, P.;Erjavec, B.;Pintar, A. Chem. Eng. J.2012,207–208, 299–307.
- Barroso-Quiroga, MM.; Castro-Luna, AE.Int. J. Hydrogen Energy,2010,35, 6052–6.
- Rivas, JL.; Fierro, MR.;Goldwasser, PE.; Pérez-Zurita, MJ.;Griboval-Constant, A.;Leclercq, G.Appl. Catal. A2008, 344, 10–19.
- Sierra Gallego, G.;Mondragón, F.;Barrault, J.;Tatibouёt, JM.;Batiot-Dupeyrat, C. Appl. Catal. A: Gen.2006,311, 164–71.
- Moradi, GR.; Rahmanzadeh, M.Catal. Commun.2012, 26, 169–72.
- Gallego German, S.; Marin Jaime, G.; Batiot-Dupeyrat, C.; Barrault, J.; Mondragon, F. Appl. Catal A: Gen.2009,369, 97-103.
- Lima, SM.;Assaf, JM.; Pena, MA.;Fierro, JLG.Appl. Catal. A: Gen.2006,311, 94-104.
- Choi, So.; Moon, SH. Catal. Today,2009,146, 148-53.
- Lima, SM.; Silva, AM.; Costa, LOO.;Assaf, JM.;Mattos, LV.;Sarkari R. et al. 2012 , 121-122, 1-9.
- Sutthiumporn, K.;Maneerung, T.; Kathiraser, Y.; Kawi, S.Int. J. Hydrogen Energy.2012, 37, 11195-207.
- Jahangiri, A.;Pahlavanzadeh, H.; Aghabozorg, HR. Int. J. Hydrogen Energy.2012,37, 9977-9984.
- Jahangiri, A.; Aghabozorg, HR.; Pahlavanzadeh, H.Int J Hydrogen Energy.2013, 38, 10407-10416.
- Jahangiri, A.; Aghabozorg, HR.; Pahlavanzadeh, H.; Towfighi, J.Int. J. Chem. React. Eng.2014, 12, 1–10.
- Dejaidja, A.; Libs, S.;Kiennemann, A.; Barama, A.Catal. Today.2006,113(3), 194-200.
- Lima, SM.;Assaf, JM.;Pen˜ a, MA.;Fierro, JLG.Appl. Catal. A: Gen.2006, 311, 94-104.
- Pecchi, G.; Reyes, P.; Zamora,R.; Cadu´ s,L.E.;Fierro,J.L.G.J. Solid State Chem.2008,181 (4), 905-912.

This work is licensed under a Creative Commons Attribution 4.0 International License.









