Composition of Volatile Oil and Methanolic Extract of Jordanian Melissa Officinals L. and Actions Againsthuman Cancer Cell Lines
Salem A. Barakat1, M. Hudaib2, Noor EL-asadand2 and D. T. Burns3
1Department of Chemistry.Jordan University of Science and Technology, 22110 Irbid Jordan.
2Department of Pharmacy(b), Jordan University, Jordan.
3The Institute of Global Food Security, The Queen’s University of Belfast, Belfast, BT9 5HN, Northern Ireland, United Kingdom.
Corresponding Author E-mail: Barakat@just.edu.jo
DOI : http://dx.doi.org/10.13005/ojc/320506
The essential oil of Jordanian MelissaofficinalisL. were obtained by hydro-distillation and analyzed by Gas Chromatography – Mass Spectrometry. Components representing 96.40% of the total oil were identified. The methanolic extract and the volatile oil of Melissa officinalisL, were tested and showed anti-proliferation activities against 3 cancer cell lines.
KEYWORDS:Melissa officinalis. Methanolic extract, GC-MS
Download this article as:| Copy the following to cite this article: Barakat S. A, Hudaib M, EL-asadand N, Burns D. T. Composition of Volatile Oil and Methanolic Extract of Jordanian Melissa Officinals L. and Actions Againsthuman Cancer Cell Lines. Orient J Chem 2016;32(5). |
| Copy the following to cite this URL: Barakat S. A, Hudaib M, EL-asadand N, Burns D. T. Composition of Volatile Oil and Methanolic Extract of Jordanian Melissa Officinals L. and Actions Againsthuman Cancer Cell Lines. Orient J Chem 2016;32(5). Available from: http://www.orientjchem.org/?p=21750 |
Introduction
Most people living in less developed countries rely almost exclusively on traditional medicines for their healthcare needs. In Jordan also many people use herbal medicines as alternative, additional or complementary medicine [1-2]. However, most of the plants used in traditional medicine in Jordan lack detailed phytochemical study and biological evaluation [3].
One of the most interesting medicinal plant species in Jordan is Melissa, a genus of the madder family Labiatae [4]. It is widely cultivated in Europe and the United States [5].There has been considerable interest in the biological effects of essential oils from a variety of plants [6] and in their antimicrobial [7] and antioxidant properties [7-9]. The content and composition of the oil Melissa officinalisvary with its origin within a given country [10], from country to country [11-13], and under the influence of nitrogenous fertilizers [14] and growth regulators [15]. This variability increases the importance of the study of a wide range of Melissa samples. In addition to themelissa essential oil composition, the aromatic and polyphenolic composition of herbal tea made from lemon balm (Melissa) has been reported [16].
Malissa has traditionally been used to treat a wide range of conditions such as fever, flatulence, headache, influenza and toothache [2, 5]. Numerous specific biochemical activities have been reported [17] such as acetylcholinesterase inhibition and antioxidant activity [18] and its use for the treatment of cancer [19, 20] and of diabetes [21] have been evaluated.
Milessa occurs rarely in Jordan but is found in restricted regions at WadiRajeb. It is a neglected, underutilized plant and threatened by wild herbs. Preliminary work was carried out by Syouf with collections from 3, not previously, studied sites in Jordan [22] the locations are shown in Table 1-1.
Table 1: Collection of wild Melissa officinalisfrom 3 sites in Jordan, with GIS data.
|
Longitude E |
Latitude N |
Elevation |
JO. NO. |
Collection location |
Number |
|
35 41 50.0 |
32 18 23.5 |
506 |
3098 |
Kufrangeh |
1 |
|
35 41 54.1 |
32 14 31.5 |
300 |
3099 |
WadiRajib |
2 |
|
35 41.33.3 |
32 13 59.5 |
418 |
3100 |
Rajib ( Al Tal) |
3 |
The essential oil composition of Melissa from Jordan and its anti-cancer proliferative activity have not been studied previously. Here in the analysis of the essential oil from these Melissa species is described for the first time.
Material and Method
Plant materials
A large sample of wild Melissa officinalis SP were collected in Jordan in 2011. The plants were identified by Dr. Maha Al- Syuof. (Biodiversity department, NCARE[22]) .The aerial parts of the plants were dried at room temperature and then coarsely powdered.
Extraction, isolation and identification of the essential oil
Dried leaves of M. officinaliswere subjected to hydrodistillation for 3 hours using
a Clevenger type apparatus [23]. The oil was collected, dried over anhydrous sodium sulphate and stored in the dark in a refrigerator until analyzed.
Gas Chromatographic – Mass Spectral (GC-MS) Analysis
About 1 μl aliquot of each oil sample, diluted in n-hexane, was subjected to GC-MS analysis. GC-MS analysis was performed using a Varian Chrompack CP-3800 GC/MS/MS-200 (Saturn, Netherlands) equipped with split-splitless injector and DB-5 (5% diphenyl, 95% dimethylpolysiloxane) capillary GC column (30m x 0.25mm ID, 0.25 μl film thickness). The carrier (ultra-pure helium) flow rate was 1ml/min. The column temperature was kept at 100ᵒC for 3 min and then programmed at rate of 10ᵒC/min up to 250ᵒC, and then held at 250ᵒC for 60 min. The total run time was 56.98 min the mass detector was set to scan ion between 35-500 m/z. A mixture of n-alkanes (C8-C20) was analyzed separately under the same conditions using the same DB-5 column. The compounds in the volatile oil s were identified, using built in libraries (NIST Co and Wiley Co, USa)
Identification of the compounds
The compounds were identified by comparing the retention time, retention index and mass spectrum of the chromatographic peaks with that of the standardsavailable .
The identification of other components was by computer matching with the Wiley, NIST and ADAMS libraries [24] based on their retention indices [25] determined by reference to a homologous series of n-alkanes, (C8-C20) and by comparison of their mass spectral fragmentation patterns with those reported in the literature [24] and stored on the MS library data system. Using the van den Dool equation [26], to get RI values helps by predicting the closet component from the top ten component summary which are given from the data system.
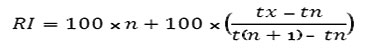
tx = retention time of unknown component
tn = retention time of preceding n-alkane
t(n+1) = retention time of following n-alkane
n = carbon number of preceding n-alkane
RI: arithmetic index as reported in literature .
KI: Kováts index as reported in literature .
Results
In the Table(1-2).which follow the major oil components (component %>3) are indicated in bold.
Table 2: the chemical composition for the volatile oil of Melissa officinalis SP.
| No |
Rt |
Content |
AI |
KI |
Compounds |
|
MEL98R22 LEV |
|||||
|
1 |
10.523 |
0.513 |
1044 |
1050 |
<(E)-B>OCIMENE |
|
2 |
13.956 |
0.109 |
1122 |
1126 |
<alpha->Campholenal |
|
3 |
15.567 |
0.331 |
1160 |
1164 |
Pinocarvone |
|
4 |
16.320 |
1.659 |
1174 |
1177 |
Terpinen-4-ol |
|
5 |
19.149 |
0.331 |
1238 |
1241 |
Cumin aldehyde |
|
6 |
21.323 |
0.010 |
1289 |
1290 |
Thymol |
|
7 |
22.899 |
0.401 |
1325 |
1327 |
<p->Mentha-1,4-diene-7-ol |
|
8 |
24.653 |
2.516 |
1374 |
1376 |
<alpha->copaene |
|
9 |
24.999 |
3.141 |
1387 |
1388 |
<beta->Bourbonene |
|
10 |
25.225 |
2.724 |
1381 |
1382 |
Panasinsene |
|
11 |
26.572 |
3.417 |
1417 |
1419 |
<(E)->Caryophellene |
|
12 |
28.053 |
1.931 |
1449 |
1415 |
Amorpha-4,11-diene |
|
13 |
29.089 |
28.847 |
1478 |
1479 |
<Gamma->Muurolene |
|
14 |
31.850 |
3.530 |
1548 |
1549 |
Elemol |
|
15 |
33.166 |
43.556 |
1582 |
1583 |
Caryophellene Oxide |
|
16 |
34.128 |
2.006 |
1608 |
1608 |
Humulene epoxide II |
|
17 |
36.435 |
1.431 |
1680 |
1680 |
Elemol acetate |
|
18 |
42.000 |
2.471 |
disappear |
||
|
MEL98R22 ST |
|||||
|
1 |
10.557 |
0.1 |
1044 |
1050 |
<(E)-B>OCIMENE |
|
2 |
12.183 |
0.1 |
1086 |
1088 |
<alpha->Terpinolene |
|
3 |
14.551 |
0.2 |
1135 |
1139 |
<trans->pinocarveol |
|
4 |
15.367 |
trace |
1160 |
1164 |
<cis->Chrysanthenol |
|
5 |
16.321 |
0.3 |
1174 |
1177 |
Terpinen-4-ol |
|
6 |
19.126 |
trace |
1238 |
1241 |
Cumin aldehyde |
|
7 |
20.428 |
trace |
1264 |
1267 |
E-citral |
|
8 |
21.309 |
trace |
1298 |
1299 |
Carvacrol |
|
9 |
22.496 |
0.2 |
1315 |
1316 |
<(2E,4E)->Decadienal |
|
10 |
24.648 |
2.1 |
1374 |
1376 |
<alpha->copaene |
|
11 |
24.998 |
2.0 |
1387 |
1388 |
<beta->Bourbonene |
|
12 |
25.221 |
20.9 |
1381 |
1382 |
Panasinsene |
|
13 |
26.508 |
15.8 |
1417 |
1419 |
<(E)->Caryophellene |
|
14 |
28.047 |
4.5 |
1449 |
1415 |
Amorpha-4,11-diene |
|
15 |
29.023 |
22.2 |
1478 |
1479 |
<Gamma->Muurolene |
|
16 |
31.847 |
trace |
1548 |
1549 |
Elemol |
|
17 |
33.060 |
30.5 |
1582 |
1583 |
Caryophellene Oxide |
|
18 |
41.041 |
0.2 |
1800 |
1800 |
isotorquatone |
|
19 |
42.409 |
0.4 |
Unkown |
||
| Normal Monoterpenes |
0.2% |
||||
| Oxygenated Monoterpenes |
2.6% |
||||
| Normal sesquiterpenes |
39.1% |
||||
| Oxygenated sesquiterpenes |
53.9% |
||||
Table 3: Major component in Melissa officinalissp
| 98R22 Leaf | Major Compound |
%Content >3 |
| 1 |
<(E)->Caryophellene |
3.4 |
| 2 |
<beta->Bourbonene |
3.1 |
| 3 |
<Gamma->Muurolene |
28.8 |
| 4 |
Elemol |
3.530 |
| 5 |
Caryophellene Oxide |
43.556 |
| 98R22 Stem |
Major Compound |
Content >3% |
| 1 |
Panasinsene |
20.9 |
| 2 |
<(E)->Caryophellene |
15.8 |
| 3 |
Amorpha-4,11-diene |
4.5 |
| 4 |
<Gamma->Muurolene |
22.2 |
| 5 |
Caryophellene Oxide |
30.5 |
The total amount content was 0.1 ml.
The Chemical composition of Melissaofficinalis from WadiRujb /Ajlun is shown in Table 1-4.
Table 4: Chemical composition of Melissaofficinalis SP WadiRujb /Ajlun.
The amount of oil collected was 0.2 ml.
|
No |
Rt |
Content |
AI |
KI |
MEL99R31LEV |
|
1 |
10.112 |
0.1 |
1032 |
1037 |
<(Z)-B>Ocimene |
|
2 |
14.568 |
0.1 |
1137 |
1142 |
<trans->Sabinol trans for OH vs IPP |
|
3 |
15.408 |
0.1 |
1160 |
1164 |
Pinocarvone |
|
4 |
16.337 |
0.1 |
1174 |
1177 |
Terpinen-4-ol |
|
5 |
18.821 |
1.6 |
1235 |
1238 |
Neral |
|
6 |
20.119 |
2.6 |
1160 |
1164 |
<(Z)->Isocitral |
|
7 |
24.642 |
3.1 |
1374 |
1376 |
<alpha->copaene |
|
8 |
24.986 |
3.2 |
1387 |
1388 |
<beta->Bourbonene |
|
9 |
25.148 |
1.1 |
1389 |
1390 |
<Gamma->Elemene |
|
10 |
26.510 |
16.4 |
1417 |
1419 |
<(E)->Caryophellene |
|
11 |
28.037 |
4.3 |
1449 |
1451 |
Amorpha-4,11-diene |
|
12 |
29.030 |
19.9 |
1478 |
1479 |
<Gamma->Muurolene |
|
13 |
31.841 |
2.4 |
1548 |
1549 |
Elemol |
|
14 |
33.092 |
39.8 |
1582 |
1583 |
Caryophyllene Oxide |
|
15 |
34.112 |
1.7 |
1608 |
1608 |
Humulene epoxide II |
|
16 |
36.425 |
2.3 |
1668 |
1669 |
<(Z)->Caryophyllene<14-hydroxy-9-EPI |
|
17 |
42.261 |
0.1 |
Unkown |
||
|
MEL99R31ST |
|||||
|
1 |
10.559 |
0.0 |
1044 |
1050 |
<(E)-Beta->Ocimene |
|
2 |
12.744 |
0.1 |
3-methyl-2-(2-methyl-2-butenyl)-furan |
||
|
3 |
14.660 |
0.1 |
1137 |
1142 |
<trans->Sabinol trans for OH vs IPP |
|
4 |
15.445 |
0.1 |
1160 |
1164 |
Pinocarvone |
|
5 |
16.280 |
0.3 |
1174 |
1177 |
Terpinen-4-ol |
|
6 |
19.564 |
0.1 |
1238 |
1241 |
Cumin aldehyde |
|
7 |
20.477 |
6.7 |
1264 |
1267 |
E-citral |
|
8 |
21.486 |
4.2 |
1299 |
1299 |
<cis-α ->Necrodol acetate |
|
9 |
24.983 |
3.8 |
1387 |
1388 |
<beta->Bourbonene |
|
10 |
25.523 |
2.2 |
1389 |
1390 |
<Gamma->Elemene |
|
11 |
26.493 |
15.6 |
1417 |
1419 |
<(E)->Caryophellene |
|
12 |
28.003 |
4.3 |
1449 |
1451 |
Amorpha-4,11-diene |
|
13 |
29.003 |
17.4 |
1478 |
1479 |
<Gamma->Muurolene |
|
14 |
33.040 |
42.6 |
1582 |
1583 |
Caryophyllene Oxide |
|
15 |
36.523 |
1.9 |
1668 |
1669 |
<(Z)->Caryophyllene<14-hydroxy-9-EPI |
|
16 |
41.035 |
0.1 |
1800 |
1800 |
Isotorqutone |
|
17 |
42.238 |
0.5 |
Unkown |
||
| Normal Monoterpenes |
3.2% |
||||
| Oxygenated Monoterpenes |
4.3% |
||||
| Normal Sesquiterpenes |
48% |
||||
| Oxygenated Sesquiterpenes |
46.3%
|
||||
The amount of oil collected was 0.2 ml.
Table 5: Major Components in the Mellissa officinalis SP
| 99R31Leaf |
Major compounds |
% Content>3 |
| 1 |
< α->copaene |
3.1 |
| 2 |
<β ->Bourbonene |
3.2 |
| 3 |
<(E)->Caryophellene |
16.4 |
| 4 |
Amorpha-4,11-diene |
4.3 |
| 5 |
<γ ->Muurolene |
19.9 |
| 6 |
Caryophyllene Oxide |
39.8 |
| 99R31 Stem |
Major compounds |
% Content>3 |
| 1 |
E-citral |
6.7 |
| 2 |
<cis-α ->Necrodol acetate |
4.2 |
| 3 |
<β ->Bourbonene |
3.8 |
| 4 |
<(E)->Caryophellene |
15.6 |
| 5 |
Amorpha-4,11-diene |
4.3 |
| 6 |
<γ ->Muurolene |
17.4 |
| 7 |
Caryophyllene Oxide |
42.6 |
Antiproliferative Activity Against Human Cancer Cell Lines
The methanolic extractof Melissa officinalis SP was tested on three cancer cell lines namely : two types of colorectal (SW480), (HCT116), and prostate (PC3).As were the volatile oils of the plants.
These cancer cell lines were treated with different concentration of plant extract
( 10µg/ml,25 µg/ml,50 µg/ml,100 µg/ml), and for the volatile oil (1 µg/ml,1.5 µg/ml,5 µg/ml, 10 µg/ml) , for 72 hr.
The cells growth were evaluated using MTT assay as illustrated in below.
Table 6: IC50 for Melissa officinals SP methanolic extract.
|
SW480 |
HCT116 |
PC3 |
|
|
0 |
100 |
100 |
100 |
|
10 |
100 |
100 |
100 |
|
25 |
100 |
100 |
100 |
|
50 |
85.51449 |
100 |
100 |
|
100 |
60.43956 |
69.11142 |
94.47674 |
|
IC50 |
130.2 |
180.68 |
H |
|
0.9459 |
0.7776 |
Table 7: IC50 for Melissa officinalis SP volatile oil.
|
SW480 |
HCT116 |
PC3 |
|
|
0 |
100 |
100 |
100 |
|
1 |
86.95652 |
100 |
100 |
|
2.5 |
80.43478 |
100 |
100 |
|
5 |
67.3913 |
100 |
100 |
|
10 |
67 |
100 |
100 |
|
IC50 |
13.67 |
H |
H |
|
R2 |
0.745 |
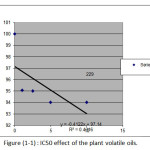 |
Figure 1: IC50 effect of the plant volatile oils. |
Table 8: IC50 (µg/mL) of different plant fractions against different cancer cell line.
|
|
SW40 |
HCT116 |
PC3 |
|
Cultivated V. Oil |
114 |
H |
H |
|
Wild V. Oil |
13.67 |
H |
H |
|
Cultivated Crud Ext. |
135 |
229 |
199.7 |
|
Wild Crud Ext. |
130.2 |
180.68 |
H |
Conclusion
Herein is reported the chemical composition of the volatile oils obtained by hydrodistillation from the two Melissa species, The plant also evaluated for its anti-proliferative activities using (SW480), (HCT116), and (PC3) , cancer cell lines. Results revealed that the methanolic extract of Mellissa officinalshasan effect in cell viability. Further studies are needed for determination of the mode of action(s) of these plants antiproliferative activities.
The present study strengthen evidence that the search for new anti cancer agent should emphasize to the screening of natural flora of the different countries.
It can be noticed generally that the chemical composition of JordanianMellissa officinlis sp ,and Mellissa officinalisin other countries have not identical components .
The composition of the oil from M. officinalis harvested in Algeria was dominated by
neral, geranial and citronellal. This composition was qualitatively the same that the oilsfrom Serbia (Dukic et al., 2004), Slovak [22], Egypt (Shalaby El-
Gengaihi and Khattab, 1995), France (Carnatet al., 1998) and Iran (Sadraei et al., 2003);
However, limonene was the major component in the samples from
Scotland (Damien et al., 2000) (57.5 %), neral was found with only (4.3 %) and geranial
was completely absent. Basta et al. (2005) reported that caryophyllene oxide (12.6 %) and β-pinene (18.2 %) were also the most abundant constituents in the oil of M. officinalisfrom Greece but neral and geranial were not detected in the oil. Oils from Cuba [13]) and Brazil [15] were dominated by neral (29.9 % and39.3 %) and geranial (41.0 % and 47.3 %) respectively.
Which clearly leads to conclusion that the chemical composition of the plantand volatile oil composition may vary according to location.
Aknowledgement
The authors aknowledge Highercounceil at Jordan University of Science& Technology for Financial support.
References
- Balunas M. J. and Kingborn, A. D. Drug discovery from medicinal plants. Life sciences.(2005). 78, 431-441.
- Al-Khalil S. A survey of Plants Used in Jordanian Traditional Medicine. Int. J. of Pharmacog.,(1995). 33, 317-323.
- Al-Eisawi D. M. H. Field Guide to Wild Flowers of Jordan and Neighbouring Countries. Jordan Press Foundation “AL RAI”, Amman, Jordan. . (1998)
- Feinbrun-Dothan N. in M. Zohary and N. Feinbrun-Dothan, (eds). Flora Palaestina, Part3. Israel Academy of Sciences And Humanities, Jerusalem, Pp.146-147
- Moradkhani H. et alia. Melissa officinals L., a valuable medicine plantA review. J. Med. Plants Res., (2010)4, 2753-2759.
- Bakkali F., Averbeck S., Averbeck D. and Idaomar M. Biological effects of essential oils-A review, Food Chem. Toxicol.,(2008). 46, 446-475.
- Mimica-Dukic N., Bozin B., Sokovic M. and Simin N. Antimicrobial and antioxidant activities of Melissa officinalis L. (Lamiaceae) essential oil. J. Agric. Food Chem., (2004) 52, 2485-2489.
- Dorman H. J. D., Surai P. and Deans S. G., In Vitro antioxidant activity of plant essential oils and phytoconstituents, J. Essent. Oil Res.,(2000)12, 241-248.
- Koksal E., Bursal E., Dikici E., Tozoglu F. and Gulcin I. Antioxidant activity of Melissa officinalis leaves, J. Medic. Plants Res.,(2011). 5, 217-222.
- Patora J., Majda T., Góra J. and Klimek B. Variability in the content and composition of essential oil from lemon balm (Melissa officinalis L.) cultivated in Poland. Acta Polonia Pharma.,(2003). 60, 395-400.
- Sari A. O., and Ceylan A. Yield characteristics and essential oil composition of Lemon Balm (Melissa officinalis L.) grown in the Aegean region of Turkey. Turk. J. Agric For.,(2002). 26, 217-224.
- Hollá M. et alia Composition of the essential oil from Melissaofficinalis L. cultivated in Slovak Republic, J. Essent. Oil Res.,(1997). 9, 481-484.
- Pino J. O., Rosado A. and Fuentes V. Composition of the essential oil of Melissa officinalis L. from Cuba, J. Essent. Oil Res., (1999).11, 363-364.
- Abbaszadeh B., Farahani H. A., Valadabadi S. A. and Darvishi H.H. Nitogenous fertilizer influence on quantity and quality values of balm (Melissa officinalis L.), J. Agric. Ext. Rural Dev.(2009), 1, 31-33
- Da Silva S. et alia Essential oil composition of Melissa officinalis L. in vitro produced under the influence of growth regulators. J. Braz.Chem. Soc., 1(2005) 6, 1387-1390.
- Carnat A. P., Carnat A., Fraisse D. and Lamaison J. LThe aromatic and polyphenolic composition of lemon balm (Melissa officinalis L. Subsp. officinalis tea. Pharma. Acta Helvet., . (1998). 72, 301-305.
- Lemon balm (Melissa officinalis), Plant profiler, Sigma-Aldrich.http://sigmaaldrich.com/life/nutrician-research/learning-centre/plant-profiler/Melissa-officinalis.html accessed 19/4.2016.
- Ferreira, A., Proenca C., Serralheiro M. L. M. and Araujo. M. E. M The in vitro screening for acetycholinesterase inhibition and antioxidant activity of medicinal plants from Portugal. J. of Ethnopharmacology, . (2006).108, 31-37.
- Afifi-Yazar F. U., Kasabri V. and Abu-Dahab R.. Medicinal plants from Jordan in the treatment of cancer: Traditional uses vs. in vitro and in vivo evaluations- part 1. Planta Med. (2011)77, 1203-1209.
- Jahanban-Esfahahlan,A., Modaeinama, S., Abasi, M., M. Abbasi, M. M. and Jahanban-Esfahahlan, R. Anti-Proliferative Properties of Melissa officinalis in Different Human Cancer Cells, Asian Pacific J. CancerPrevention,(2015).16, 5703-5707.
- Afifi-Yazar F.U., Kasabri V. and Abu-Dahab R. Medicinal Plants from Jordan in the treatment of Diabetes: Traditional uses vs. in vitro and in vivo evaluation- Part2, Planta Med.,. (2011) 77, 1210-1220.
- Syouf, Q. M.. In situ conservation of Medicinal plants in Jordan. Medicinal herbal plant project. National Center for Agricultural Research and Extension.(2007)
- Clevenger, J. F. Apparatus for the determination of volatile oil. J.Amer. Pharma. Assn.,(1928). 17, 345-349
- Adams, R. P., Identification of Essential Oils by Gas Chromatography/Mass Spectroscopy, 4th. Edn., Allured publishing, Carol stream, Illinois, USA.(2012).
- Kováts, E. Gas-chromatographische Charaktersierung organischer Verbindungen. Retenionsindices aliphatischer Halogenide, Alkohole, Aldehyde und Ketone Helv. Chim. Acta(1958). 41, 1915-1932.
- Van den Dool, H. and Kratz, P .D., A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromat.,(1963).11, 463-471.
- Shalaby, A. S., El-Gengaihi and Khattab MOil of Melissa officinalis L., as affected by storage and herb drying, J. Essent. Oil Res ,(1995) 7, 667-669.

This work is licensed under a Creative Commons Attribution 4.0 International License.









