Comparison of Conventional and Minerals, Extraction. Extraction Methods for Tio2 Recovery in Mineral Sands
Muhammad Nurdin*, Ahmad Zaeni, Maulidiyah, Muhammad Natsir, Ardianto Bampe and Dwiprayogo Wibowo
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Halu Oleo, Kendari 93232 – Southeast Sulawesi, Indonesia.
Corresponding Author E-mail: mnurdin06@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/320545
Iron sand as the source of many important minerals is one of natural resources in Southeast Sulawesi. The iron sand minerals that contain TiO2 has economic value and a number of application i.e. environmental protection, paint industries, sensor and photovoltaic. The extraction of TiO2 has been done from pseudorutile (Fe2Ti3O9) using dissolution of HCl 20% and the addition of Fe0 reductor to optimize the microwave and conventional process. The result showed the optimized yield value of TiO2 and Fe2O3 are 74.49% and 80.35%, respectively. The power used effect of microwave -assisted to the extraction process was obtained decreasing of 81%, and the extraction time could be efficiency from 6 to 2 hours. The microwave extraction process can increase a yield of TiO2 and significant decreasing the yield of Fe2O3 in a shorter time if compared to the conventional process.
KEYWORDS:iron sand; microwave; TiO2; Fe2O3; minerals; extraction
Download this article as:| Copy the following to cite this article: Nurdin M, Zaeni A, Maulidiyah M, Natsir M, Bampe A, Wibowo D. Comparison of Conventional and Minerals Extraction.Extraction Methods for Tio2 Recovery in Mineral Sands. Orient J Chem 2016;32(5). |
| Copy the following to cite this URL: Nurdin M, Zaeni A, Maulidiyah M, Natsir M, Bampe A, Wibowo D. Comparison of Conventional and Minerals Extraction. Extraction Methods for Tio2 Recovery in Mineral Sands. Orient J Chem 2016;32(5). Available from: http://www.orientjchem.org/?p=21274 |
Introduction
Titanium dioxide (TiO2) is a mineral that obtained more attention of the scientists and researchers because it has a wide range of applications. TiO2 is an inorganic material exploited widely in various industrial areas and used in many human life aspects such as in the industry of paint, porcelain, glass, paper, rubber, textile, floor layering, skin ointment and cream, the hydrogen production, and the manufacturing of self-cleaning material1-3. Recently, the important role of that oxide compound has been rapidly increasing, as it has a good semi-conductive and photosensitive ability, so that it is quite sufficient to be a solar cell material4-6. One of the applications of TiO2 in the control of environmental contamination is the degradation of wastewater by photoelectrocatalyst and COD sensor7-9.
TiO2material is continuously produced and developed in various methods, because of its multiplicative characteristic. The production of TiO2 can be done by using various synthetic methods. Nurdin et al. and Byranvand et al. revealed that nowadays some synthetic methods of TiO2 film had been developed, such as the method of sol-gel, hydrothermal, solvothermal, chemical vapor deposition, electrodeposition, direct oxidation (anodizing), and the others10,11. The direct oxidation method is the cheapest and the easiest of those methods. The anodizing method is developed by the scientist by using Ti plate as the basic material in developing electrode of photoelectrocatalyst and COD sensor12-15. However, the need of using Titanium metal has encouraged the scientists to develop a research in finding Titanium metal from the province of Southeast Sulawesi that is reported of having some spots of ilmenite iron sand (FeTiO3) as the source of TiO216.
The extraction of TiO2from the iron sand is an alternative solution to provide adequate and easily obtained material supply17. The presence of iron sand in nature at least can be found in four structures: ilmenite (FeTiO3), pseudorutile (Fe2Ti3O9), leucoxene and rutile (TiO2)18,19. Theoretically, ilmenite containing approximately 31% iron and 56% titanium dioxide20,21. The presence of ilmenite in nature can also easily be found and the research of this matter is broadly obtained.
Ilmenite can be naturally found in the deposit form of beach sand18,22. Mohapatra et al. explain that the main commercial source of Ilmenite all around the world is derived from black mineral sand found on the river banks or coasts18,23. Pseudorutile is the natural source of TiO2 in the form of mineral sand other than ilmenite. Pseudorutile is an intermediate product derived from the weathering process of ilmenite24-26. In other words, it is the intermediate mineral between ilmenite and rutile formed by the natural changes of ilmenite27. The method used to isolate TiO2 from the mineral sand is leaching by using acid solution28,29.
The chloride process becomes the standard process that has been universally accepted to isolate titanium from ilmenite directly through chlorination pathway30. The optimization of the chloride process is not only limited to the oxidation or reduction of the ilmenite but also on the development of extraction method31. This research on the extraction of ilmenite has been develop by using microwave-assisted method because it has the benefit of using less reagent and the shorter time of dissolution if it is compared to the conventional method.
Material and Methods
Magnetic Separation
Magnetic separation was conducted by using the magnetic separator to separate the magnetic minerals from the non-magnetic. The magnetic sand was then characterized by using XRF analyzer.
Leaching Separation
The leaching process was done by using 200 mL of 20% HCl that was heated to the temperature of 100°C. The heating of the solution was conducted in two methods. The first method (the conventional method) was used a hot plate with the temperature of 500°C and the second method was used the microwave-assisted reactor to heat the solution.
The leaching process was continued by pouring 2.5 g of iron sand into the solution and let it stand for 30 minutes, then adding 0.7 g Fe0 as a reductor and heating it for 5.5 hours in the conventional method and 1.5 hours in microwave-assisted method. After the leaching process has finished, the solution was filtered through filter paper. The residue was cleaned and weighed, then it was set to 300 rpm centrifugation and the calcinations was set at the temperature of ±550˚C that was then characterized by using XRF
Results and Discussion
The identification result of XRF magnetic sand
The characterization of the sample before extraction process was the fundamental stage related to the determination of the presence or the absence of the target material in the substance that would be identified. The magnetic sand used in this research was characterized by using XRF to identify TiO2 and other compounds and their percentage in the sample. Table 1 shows the XRF characterization result of the magnetic sand compounds value found in the province of Southeast Sulawesi.
Table 1: The value and composition of magnetic sand.
|
Compounds |
Mass Fraction (%) |
|
Fe2O3 |
61.69 |
|
TiO2 |
22.82 |
|
SiO2 |
8.78 |
|
CaO |
3.69 |
|
MnO |
1.56 |
|
Cl |
0.6 |
|
K2O |
0.219 |
|
P2O5 |
0.157 |
|
ZrO2 |
0.077 |
|
ZnO |
0.0712 |
|
Nb2O5 |
0.0406 |
|
SrO |
0.0162 |
|
SnO2 |
0.008 |
|
In2O3 |
0.0061 |
According to the Table 1, it can be seen that the compounds found to be the most in the magnetic sand were Fe2O3 dan TiO2 with the percentage of 61.69% and 22.82%, respectively. Regarding the fact that Fe found in the iron sand from Tapunggaya sub-district was Fe2O3, it could be concluded that the material was not ilmenite (FeTiO3), but it was indicated to be pseudorutile (Fe2Ti3O9). Besides those two primary data, it could also be known that the impurity compound with the highest value was SiO2 with the percentage of 8.78%.
Mechanism of Dissolution and Reduction of the Iron
The extraction principle of TiO2 from the iron sand used HCl solvent by dissolving as many iron oxides as possible that were found in the sample so that the sediment with the high value of TiO2 could be obtained. The mechanism of reaction suggested was as follows32:
Fe2Ti3O9 + 12HCl→ 2FeCl3+ 3TiOCl2+ 6H2O………………………………………………………. (1)
According to the equation (1), the forming solution was FeCl3 with the typical color of reddish orange corresponds to the color of a solution obtained in this research, as can be seen in Fig. 1.
 |
Figure 1: The solution of FeCl3 derived from the leaching process of HCl
|
The addition of Fe0 as a reductor had an important role in maximizing the process of Fe2O3dissolution because the Fe3+ in thecompound was difficult to dissolve33,34. Fe0 that is addedat the leaching process had the function of reducing Fe3+ to be Fe2+ which is easier to dissolve in HCl. The mechanism of reaction suggested for that process was as follows31:
Fe0 + 2HCl → FeCl2+H2…………………………………………………………………………………….. (2)
Fe0 + 2FeCl3→ 3FeCl2………………………………………………………………………………………… (3)
Fe0 +2TiOCl2+ 4HCl → 2TiCl3+ FeCl2+ 2H2O……………………………………………………… (4)
2TiCl3 + Fe2O3 + 2HCl → 2TiOCl2 + 2FeCl2 + H2O………………………………………….. (5)
TiOCl2 + H2O → TiO2 ↓ + 2HCl………………………………………………………………………… (6)
The mechanism of reaction above shows that the addition of Fe0 maximized the dissolution of Fe and increased the value of TiO2 that was obtained because the final stage of the reaction mechanism (6) was the hydrolysis of TiOCl2 to be the sediment TiO2. The yield obtained after the extraction process was as weight as 0.46 g for conventional heating and 0.57 g for microwave heating.
XRF Characterization
The compounds obtained from the conventional and the microwave-assisted extraction methods had been analyzed by using XRF. This process aimed how much the value of Fe2O3 that was eliminated in the sample and how many percentages of the increasing value of TiO2 in the sample. The extracted compound of XRF identification can be seen in Table 2.
Table 2: The Result of XRF identification before and after the extraction process.
|
Compounds |
Mass Fraction (%) |
||
|
Raw Materials |
Conventional Process |
Microwave Process |
|
|
Fe2O3 |
61.69 |
10.24 |
12.12 |
|
TiO2 |
22.82 |
35.61 |
39.82 |
|
SiO2 |
8.78 |
51.66 |
46.1 |
|
CaO |
3.69 |
0.7 |
0.606 |
|
MnO |
1.56 |
0.146 |
0.264 |
|
Cl |
0.6 |
– |
– |
|
K2O |
0.219 |
0.36 |
– |
|
P2O5 |
0.157 |
0.605 |
0.489 |
|
ZrO2 |
0.077 |
0.0079 |
0.117 |
|
ZnO |
0.0712 |
– |
– |
|
Nb2O5 |
0.0406 |
0.0413 |
0.051 |
|
SrO |
0.0162 |
0.0129 |
0.0132 |
|
SnO2 |
0.008 |
– |
– |
|
In2O3 |
0.0061 |
– |
– |
The result obtained from the microwave and conventional process shows the reducing value of Fe and the significant increasing value of Ti but the very high value of SiO2 in the result of extraction affected the purity of TiO2extract.
Effectiveness of Microwave-Assisted in Optimizing the Extraction Process
The main purpose of utilizing microwave process was to decrease the energy consumption as low as possible. The conventional process used the thermal conductivity to heat the target material, the reactor had to heat the other material around the first target so that the target could be heated. It caused the high consumption of energy in the conventional system. The microwave process of target material was obtained from the internal friction happened in the target material as the effect of the heat from the microwave.
In this research, it was obtained to what extent the effects of microwave-assisted to lower energy consumption used in the extraction process. The result was obtained by comparing the process using conventional and microwave regarding the use of energy and the time allocation can be seen in Fig. 2.
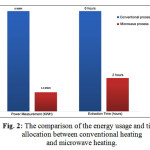 |
Figure 2: The comparison of the energy usage and time allocation between conventional heating and microwave heating. |
The process using conventional heating needed 100% power of the total output of heater (1000 watt) and 6 hours of extraction time, whereas the process using microwave heating only needed 60% of total output of the reactor so that it only needs 570 Watts. The strength of microwave heating became more visible on the time allocation for the leaching process: only 2 hours, compared to 6 hours in conventional heating.
The use of energy and time was calculated, then the total consumption of the energy obtained was 6 KWH for conventional heating and 1.14 KWH for microwave heating. This result shows that the energy consumption could be reduced approximately 81% by using microwave-assisted method.
The Effectiveness of Microwave Assisted Method
The success of TiO2extraction process from the iron sand could be seen from the value of iron oxide that could be eliminated from the material and the ability of the process in maintaining the quantity of TiO2. The extraction of TiO2 through the chloride process focused on the ability of the solvent to dissolve as many iron oxides as possible and as little TiO2as possible.
Optimization of Fe2O3Dissolution
The microwave heating was proven to accelerate and increase the dissolution process of Fe in HCl. The leaching process using microwave heating shows the significant optimization result. The result of this research can be seen in Fig. 3.
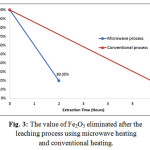 |
Figure 3: The value of Fe2O3 eliminated after the leaching process using microwave heating and conventional heating.
|
There was 80.35% of Fe2O3 eliminated during 2 hours leaching process, whereas 6 hours conventional heating could eliminate 83.4% Fe2O3. Regarding the value of Fe eliminated, there was no significant difference between the microwave heating and the conventional one. However, there were significant differences in time allocation and the energy usage between microwave heating and conventional heating.
Optimization of increasing the value of TiO2
The increasing of the TiO2 value in the extraction result by using microwave method and conventional method showed the significant percentage difference; the value of TiO2 extraction using microwave method increased 74.49%, and the value of TiO2 extraction using conventional method increased 56.04%. The increasing value of TiO2 was higher in the microwave heating in optimizing the result of extraction. It became more valuable if it also observed the shorter dissolution time of microwave heating, compared to the conventional one. Fig. 4 shows the chart of the TiO2increasing value after the extraction process using microwave heating and conventional heating.
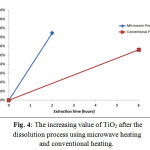 |
Figure 4: The increasing value of TiO2 after the dissolution process using microwave heating and conventional heating.
|
According to the fact shown in Fig. 4, it could be concluded that the extraction process using microwave heating was more efficient. It could be seen from the time, total energy needed, and the increasing value of TiO2.
Optimization of Purifying TiO2
The quite good result could also be obtained by the purifying process of TiO2 through the microwave heating as can be seen in Fig. 5 below.
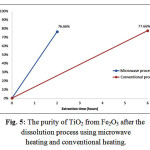 |
Figure 5: The purity of TiO2 from Fe2O3 after the dissolution process using microwave heating and conventional heating.
|
According to Fig. 5, during 2 hours of extraction process using microwave heating, the purity of TiO2 from Fe2O3 was reached 76.66%. That value of purity was nearly similar to the value obtained through conventional heating during 6 hours was reached 77.66%. The fact showed the success of the extraction process using a microwave heating in optimizing the result of extraction.
Fig. 6 shows the level of reduction of all the impurity found in the result of extraction using microwave and conventional assisted methods. Generally, the quality of the result obtained from the extraction method using microwave was better than the result obtained from the extraction process using conventional method. The total decreasing value of impurity using microwave heating was 21.75%, whereas in the method using conventional heating was 16.98%. The percentage of impurity was caused by the high value of SiO2 that was 46.1% in the result of extraction using microwave and 51.66% in the result of extraction using conventional method.
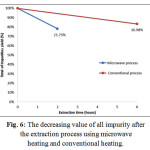 |
Figure 6: The decreasing value of all impurity after the extraction process using microwave heating and conventional heating. |
The XRF data analysis value of TiO2 and SiO2in the raw material were 22.82% and 8.78%, respectively, which explained that the ratio between TiO2 and SiO2 nearly reach 3:1. However, after the extraction process was conducted, the ratio changed drastically to 1:1. According to the fact that the value of Fe2O3haddecreased the quantity of TiO2 also decreased drastically. The decreasing quantity of TiO2 in the result of extraction had some possible causes. The first possibility was that the size of TiO2 in the material was too small so that there were some particle of TiO2passing through the filter paper and was being dumped with the filtrate during the screening process. The second possibility was that the high concentration of the solvent make the TiO2 dissolved and finally was also dumped with the filtrate.
The second possibility has been reported by Mehdilo et al. who described that the high concentration of acid used in the extraction process would directly proportional to the percentage of dissolved Fe, Mn and Ti34. The use of HCl 8 M affected more significantly to the dissolution of Fe, but the Ti dissolution also increased. This condition was less favorable for the extraction process that needed a product having high TiO2 on the solid phase because of the high value of dissolved Ti, so that it decreased the synthetic TiO2 formed.
The value of impurity other than SiO2in the result of extraction obtained from the process using microwave heating and conventional heating had significantly decreased become 79.33% and 82.1%, respectively. It can be seen in Fig. 7.
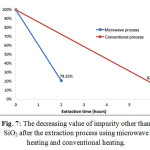 |
Figure 7: The decreasing value of impurity other than SiO2 after the extraction process using microwave heating and conventional heating.
|
Separation from the high value of SiO2 still remained in the result of extraction using the two methods provided, the extraction process using microwave heating kept showing the significant effect in optimizing the process and result of extraction. Some variables optimized by the microwave heating were the reaction rate, TiO2 increasing yield, and the decreasing number of impurity which were better than those in conventional heating. Lewicka et al. explained that by using microwave radiation has the strength in the process of eliminating Fe, considered from the reaction kinetics and the quality of the products which were better than the result obtained from the conventional heating36.
The ability of microwave heating in optimizing the process and result of extraction was about the effect of microwave heating to the mineral. The main advantage of the microwave heating emitted energy would spread evenly and quickly into the entire volume of material then heat it directly without conducting the heat. The other effect of the heating system was an occurrence of cracks in the material caused by the exposure of microwave radiation which then results to the extended surface area of the material. Those advantages of microwave heating would not be found in the conventional heating, and it was assumed as the causative factor of the reaction rate, purity of the sample, and the efficiency of energy and time. The result of microwave heating optimization entirely is presented in Fig. 8.
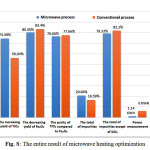 |
Figure 8: The entire result of microwave heating optimization
|
Conclusion
Based on this research, it can be concluded that the extract of TiO2 from the iron sand could be optimized well using the microwave-assisted. It had an effect on the significant energy efficiency 1.4 KWH and time allocation needed 2 hours for the extraction process showing 81% decreasing of the energy usage compared to the process using the conventional heating. The use of microwave heating had an effect on the optimized value of TiO2 and better purity than conventional heating with the percentage yield of TiO2value were 74.49% and 56.04%, respectively. While, the decreasing value of impurity on microwave method and conventional method were 20.66% and 16.59%, respectively.
Acknowledgement
We acknowledge the financial support of the DRPM– Ministry of Research, Technology and Higher Education, the Republic of Indonesia.
References
- Sethi, D.; Jada, N.; Ramasamy, S.; Pandey, S.; Kalidoss, J.; Mukherjee, P.S.; Tiwari, A. J. Photochem. & Photobio. B. 2014, 140, 69-78.
CrossRef - Maulidiyah; Nurdin, M.; Widianingsih, E.; Azis, T.; Wibowo, D. ARPN J. Engin. & Appl. Sci. 2015, 10, 6250-6256.
- Maulidiyah; Ritonga, H.; Faiqoh, C.E.; Wibowo, D.; Nurdin, M. Biosci. Biotech. Res. Asia 2015, 12, 1985-1989.
- Umar, A.A.; Nafisah, S.; Saad, S.K.Md.; Oyama, M. Solar Energy Mat. & Solar Cell. 2014, 122, 174-182.
- Maulidiyah; Wibowo, D.; Hikmawati; Salamba, R.; Nurdin, M. Orient. J. Chems. 2015, 31, 2337-2342.
CrossRef - Shah, A.A.; Umar, A.A.; Salleh, M.M. Electrochimica Acta 2016, 195, 134-142.
CrossRef - Nurdin, M.; Wibowo, W.; Supriyono; Febrian, M.B., Surahman, H.; Krisnandi, Y.K.; Gunlazuardi J. Makara, Sains, 2009, 13, 1-8.
- Maulidiyah; Nurdin, M.; Erasmus; Wibowo. D.; Natsir, M.; Ritonga, H.; Watoni, A.H. Int. J. ChemTech Res. 2015, 8(1):416-423.
- Maulidiyah; Ritonga, H.; Salamba, R.; Wibowo, D.; Nurdin M. Int. J. ChemTech Res. 2015, 8, 645-653.
- Nurdin, M.; Maulidiyah. Int. J. Sci. & Tech. Res. 2014, 3, 122-126.
- Byranvand, M.M.; Kharat, A.N.; Fatholahi, L.; Beiranvand, Z.M. J. Nanostruc. 2013, 3, 1-9.
- Ruslan; Wahab, A.W.; Nafie, N.L.; Nurdin, M. Int. J. Sci. & Tech. Res. 2013, 2, 220-224.
- Maulidiyah; Nurdin, M.; Wibowo, D.; Sani, A. Int. J. Pharm. & Pharm. Sci. 2015, 7, 141-146.
- Nurdin, M.; Muzakkar, M.Z.; Nurjannah; Maulidiyah; Wibowo, D. J. Mater. Environ. Sci. 2016, 7, 3334-3343.
- Nurdin M. Int. J. Pharma & Bio Sci. 2014, 5, 360-369.
- Nurdin, M.; Maulidiyah; Watoni, A.H.; Abdillah, N.; Wibowo, D. Int. J. ChemTech Res. 2016, 9, 483-491.
- Paul, B.; Nesterenko, P.; Nurdin, M.; Haddad, P.R. Anal. Commun. 1998, 35, 17-20.
CrossRef - Mohapatra, S.; Behera, P.; Das, S.K. J. Geosci. & Environ. Protec. 2015, 3, 31-37.
CrossRef - Bruckard, W.J.; Pownceby, M.I.; Smith, L.K.; Sparrow, G.J. Min. Proc. & Extra. Metall. 2015, 124, 47-63.
CrossRef - McKinney, R.M. Cyclic Process for the Production of Titanium Dioxide. 1965, U.S. Patent: 3,171,719.
- Pollard, L.J.; Templestowe, L.; Stewart, D.F.; Doncaster. Veneficiation of the Non-Ferrous Metal Values of Oxide-Containing Materials. 1977, U.S. Patent: 4,047,934.
- Jia, L.; Liang, B.; Lu, L.; Yuan, S.; Zheng, L.; Wang, X.; Li, C. Hydrometall. 2014, 150, 92-98.
CrossRef - Abdel-Karim, A-A.M.; Zaid, S.M.; Moustafa, M.I.; Barakat, M.G. J. African Earth Sci. 2016, 123, 10-20.
CrossRef - Teufer, G.; Temple, A.K. Nature 1966, 211, 179-181.
CrossRef - Grey, I.E.; Watts, J.A.; Bayliss, P. Mineralogical Magazine 1994, 58, 597-600.
CrossRef - Grey, I.E.; Li, C. Mineralogical Magazine 2003, 67, 733-747.
CrossRef - Qamar, M.; Merzougui, B.; Anjum, D.; Hakeem, A.S.; Yamani, Z.H.; Bahnemann, D. Catall. Today 2014, 230, 158-165.
CrossRef - Nayl, A.A.; Aly, H.F. Hydrometall. 2009, 97, 86-93.
CrossRef - Smith, Y.R.; Raj, K.J.A.; Subramanian, V.(R.); Viswanathan B. Coll. & Surf. A: Physcochem. & Engin. Aspects 2010, 367, 140-147.
CrossRef - Mehdilo, A.; Irannajad, M.; Rezai, B. Periodico di Mineralogia 2015, 84, 289-302.
- Sarker, M.K.; Rashid, A.K.M.B.; Kurny, A.S.W. Int. J. Miner. Process 2006, 80, 223-228.
CrossRef - Yarkadas, G.; Toplan, H.O.; Yildiz, K. SAU. Fen Bilimleri Dergisi 2009, 13, 18-21.
- Chirita, M.; Grozescu, I. Chem. Bull. Politehnica Univ. (Timispoara) 2009, 54,1-8.
- Liu, Q.; Dai, Y.; Luo, Y.; Chen, Y. J. Engin. Fibers & Fabrics 2016, 11, 17-25.
- Mehdilo, A.; Irannajad, M.; Rezai, B. Coll. & Surf. A: Physcochem. & Engin. Aspects 2013, 428, 111-119.
CrossRef - Lewicka, K.; Siemion, P.; Kurcok, P. Int. J. Polymer Sci. 2015, 2015, 1-11.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









