Skin and Liquid Crystal: A brief review on their similarities
Hamdan Suhaimi* and Laili Che Rose
School of Fundamental Science, Universiti Malaysia Terengganu, UMT 21030, K. Terengganu, Terengganu, MALAYSIA.
Corresponding Author E-mail: hamdans@umt.edu.my
DOI : http://dx.doi.org/10.13005/ojc/320434
Looking oneself directly into a mirror and what does one see? The answer is of course one’s face. Taking good care of ones skin is synonymous to beauty and healthy life style. However, beauty last only as thick as a skin layer so the saying goes. While, liquid crystal, as the name implies, it looks like a crystal but flow like a liquid. Thus, how doesthis stucture called liquid crystal relate to skin and beauty? As most of us are aware skin and liquid crystalall around us, be it naturally or in technological applications. Our body body are covered with skin and liquid crystals. Surprise as it may seem but that is a fact, albeit peculiar to some. Classic example of natural liquid crystals are protein and cell membranes, while in industrial application such as electronic devices,for instance, screen of our laptop, digital watches and latest application in cosmeceuticals that isliquid crystal crystal emulsion. The question now arise is how does skin relate to liquid crystal? Why is it that this structure is crucial to the structure and function of skin? How does it relate to the delivery system of active ingredients which is important in many cosmeceuticals product? This paper will provide a brief review on the relationship of these two entities and present some work done in this area of interest. A model for the lipids of the top most layer of the skin namely stratum corneum will be highlighted.
KEYWORDS:Skin; stratum corneum; liquid crystal; surfactant
Download this article as:| Copy the following to cite this article: Suhaimi H, Rose L. C. Skin and Liquid Crystal: A brief review on their similarities. Orient J Chem 2016;32(4). |
| Copy the following to cite this URL: Suhaimi H, Rose L. C. Skin and Liquid Crystal: A brief review on their similarities. Orient J Chem 2016;32(4). Available from: http://www.orientjchem.org/?p=20938 |
The Structure of Skin The skin (Figure 1a) together with its derivatives such as glands, nails, hair and other structures are termed as the Integumentary System. This integument is the skin covering the body which is in direct contect with the external environment and serves as a protective layer of the body. It is also known as the cutaneous membrane. Being a boundary between the internal body and outside system, it is subjected to wear and tear, cuts and bruises. It acts as a barrier to water in the body that might leak out or from hazardous materials that might penetrate into the body.It is also can be an indicator of the health and character of a person.
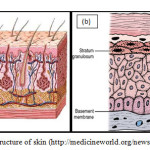 |
Figure 1. The structure of skin (http://medicineworld.org/news/news-archives) |
Traditionally, skin or integument is divided into two major layers. The outer covering layer is called the epidermis. While the inner layer is called the dermis. The dermis is made up of the papillary layer at the top and the reticular layer at the bottom. Between these layers there exist plethora of structures interchilating and crossing with each other. Beneath the skin is the subcutaneous tissue or hypodermis layer. These three layers differ in thickness in different parts of the body.As a way of illustration, in order to appreciate the effect of the dimension of size skin, the size of skin can be visualised as thick as a piece of A4 paper. Depending on the type of skin, there are 4 or 5 layers within the epidermis as shown inFigure1b. These sublayers are stratum basale, stratum spinosium, stratum granulosum, stratum lucidum (only in thick skin) and stratum cornem. The principle cell type in the epidermis is called keratinocyte and are largely found at the first three layers of the epidermis. These keratinocytes from the deepest layer of the epidermis namely the stratum basale, undergo cell division by mitosis and thus provide a continuous supply of new cells. As a result of the division these cells are being pushed upwards towards the surface and finally being shed away from the surface after a certain period.
Stratum Corneum
The shape of the dividing cell changes as they are being pushed upwards by the newer cell. It gradually becomes flattened at the subsequent layers which are then are filled with dead or the nondividing keratinocytes. Finally, as a consequece of this continuous division, a strata of about 20-30 layers of interlocking keratinized cell which are are 0.5 μm in thickness and 30-40 μm wide1. This layer is called stratum corneum. In order to complete the process, desquamation must take place. At this stage, the cells are shed away from the skin. Normally, it takes a period of 45 to 75 days from the birth to final desquamation. Studies has shown that the presence of stratum corneum reduces the water evaporation rate by a factor of 25-502. It also prevents the uptake of water into animal body, stbilizes the body temperature and also serve as a barrier to chemical and biological attack from the environment. Vast attention has been directed to the water transport through the stratum corneum3-6. Most evidences indicate that the lipids content of the stratum corneum to be the main factor in the stratum corneum barrier to trans dermal water transport. Extensive investigations towards the individual structure of stratum corneum lipids7-9 as well as their molecular organization.
Epidermal Lipids
The structure of the lipids mixture of the stratum corneum has been a subject of tremendous investigations. Most notable are those by Elias10-11, who has traced the origin and characterized the lipids of stratum corneum. Stratum corneum can be depicted as a two-compartment system analogous to brick and mortar, in which cells can be analogized as bricks, and intercelluar lamallae to mortar. A method was developed to isolate the stratum corneum lipids by using stansted cell disrupter and proteolytic digestion12. Lipids of the stratum corneum was meticulously characterized and tabulated as in shown in Table 110.
Table 1. The characterization of human epidermal lipid10
|
|
Outer Stratum Corneum |
Abdomen |
Leg |
Face |
Plantar |
| Lipid Wt (%) Fraction |
6.5 |
4.3 |
7.2 |
2.0 |
|
| Polar Lipids |
2.3 |
4.9 |
5.2 |
3.3 |
4.5 |
| Cholesterol sulfate |
3.4 |
1.5 |
6.0 |
2.7 |
3.4 |
| Neutral LipidsFree SterolsFree fatty acidsTriglyceridesNonpolar NLsSterol/was esters Squalenes n-alkanes |
68.4 18.4 15.6 11.2 19.4 – – – |
77.7 14.0 19.3 25.2 16.3 – – – |
65.7 20.1 12.7 20.1 11.7 – – – |
66.4 17.3 19.7 13.5 15.9 6.0 6.9 2.8 |
63.9 23.1 20.0 8.5 12.3 6.4 3.8 2.1 |
| SpingolipidsGlycolipids-1Glycolipid-2CeramidesCeramides/NLs |
26.6 |
18.1 |
25.9 |
26.5 |
30.1 |
It shows that the total lipid content varies at different part of the body and it also displays abundant ceramides, free sterols and fatty acids. Based on the lipids found in the stratum corneum, Elias postulated that the lipids would form a bilayer. This was on the basis that ceramides and fatty acids having polar and nonpolar character. Figure 2 shows the arrangement of lipids as postulated by Elias13.
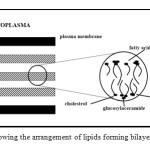 |
Figure 2: Diagram showing the arrangement of lipids forming bilayers as postulated by Elias13. Click here to View table |
Liquid Crystal
Surfactant association structures have a rich literature and continually increasing. To date (Date of search October 30, 2015; Search keyword: ‘liquid crystal’), a quick search online, show 2,850,000 articles were published involving liquid crystal (http://apps.webofknowledge.com). It ranges from micellar concept by McBain to the structure of liquid crystals and the phase behaviour of surfactants systems14-16. They are many different types of liquid crystal but can be conveniently divided into two main types namely thermotropic and lyotropics. Thermotropics liquid crystals are obtained by changing the temperature, while lyotropic liuid crystal by addition of solvents to surfactant systems. In this paper, lyotropic liquid crystal will be highlighted because of its great importance in the stability of the pharmaceutical and cosmetic applications. As the name implies, liquid crystals flow like a liquid and but the molecules are oriented in a crystal-like manner. It is characterized by its long-range order and short-range disorder17. When observed under polarized microscope, different liquid crystals will exhibit signature textures as shown in Figure3.
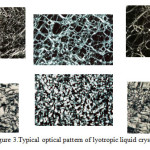 |
Figure 3: Typical optical pattern of lyotropic liquid crystals |
Different types of lyotropic liquid crystal are formed from different combinations. Interlayer spacing ranging from 10 to 100 Å are characteristic for the lyotropic liquid crystal employing low-angle x-ray diffraction. The wide-angle x-ray diffraction shows aspacing of 4.5 Å18.
Relationship Between Skin and Liquid Crystal
As postulated by Elias the lipids to be present in the stratum corneum would form bilayer13. These findings have prompted many work such as Friberg19 to investigate further. A few model epidermal lipid were formulated based on the characterization of the human stratum corneum19-21. Initial attempts to form bilayer using the model lipid mixture resulted in a completely heterogenous matrix of liquid fats, undissolved solid, and water19. Friberg then pointed out that the model would form bilayer after taking into consideration of some important factors. The most important factor is the pH of the skin22-23. The pH of a normal and healthy skin is in the range of 4.2 – 6.0. In this acidic nature of the skin, the carboxylic acids do not exist as acids but arepartially saponified. Hence, the skin conatins a mixture of soaps and the carboxylic acids. Such a mixtures has a surfactant association structure as shown in Figure4. The figure shows that, at low pH values, the combination of carboxylic acids and water, do not form any association structures. At high pH values, the acid is converted to soap and the combination of soap and water gives an isotropic micellar dispersion. At intermediate pH values, when both soap and acid are present, all three components are dispersed in a lamellar liquid crystal. Hence by neutralizing 41 percent of the free fatty acids mixture, a lamellar liquid crystal is readily formed as postulated by earlier by Elias.
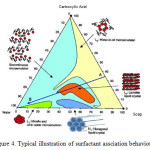 |
Figure 4: Typical illustration of surfactant assciation behaviour |
With that humble beginning, vast research has been directed towards this two entity namely skin and liquid crystal. To date (Date of search: October 30, 2015. Search keyword: ‘skin’ and “liquid crystal”), a quick search online, show 357,000 articles were published involving skin and liquid crystal (http://apps.webofknowledge.com).
References
- Plenig, G.; Marples, R.R., J Invest Dermatol.,1970, 54: 13-18.
CrossRef - A.M. Kligman; A.M. The Epidermis (W. Montgna Ed.) Academic Press, NY.1964.
- Grice, K.;Sattar, H.; Baker, H.J Invest Dermatol.,1972,58: 343-346.
CrossRef - Levequa, J.L.;Carson, J.C.; de Rigal, J.,J Soc Cosmet Chem., 1979, 30: 333-343.
- Smith, W.P.;Christensen, M.S.;Nacht, S.; Gans E.H. J Invest Dermatol.,1982. 78: 7-11.
CrossRef - Imokawa,G.;Hattori M.,J Invest Dermatol.,1985,84: 282-284.
CrossRef - Elias, P.M.; Brown B.E.; Ziboh,V.A., J Invest Dermatol.,1980, 74: 230-233.
CrossRef - Abraham,W.;Wertz, P.W.; DowningD. T., J. Lipid Res., 1985, 26(6): 761-766.
- Ranasinghe, A.W.; Wertz, P.W.; Downing D.T.; MackenzieI.C. , J Invest Dermatol1986, 86: 187-190.
CrossRef - Lampe, M. A.;Burlingame, A. L.; Whitney, J.;Williams, M.L.;Brown, B.E.;Roitman, E.; Elias,P.M., J. Lipid Res., 1983,24(2): 120-130.
- Lampe, M. A.;Williams, M. L.; Elias,P. M.J. Lipid Res., 1983,24(2): 131-140.
- Grayson S.; Elias P.M. , J Invest Dermatol, 1982, 78: 128-135.
CrossRef - Elias, P.M.;Brown, B.E.; Fritch, P.;Goerke, J.;Gray G. M.;White R.J., J Invest Dermatol.,1979, 73: 339-348.
CrossRef - McBain, J.W.;Laing, M.E.;Titley,A.F.;J Chem Soc.,1919,115: 1279-1300.
CrossRef - Luzzati, V.;Mustacchi, H.;Skoulis, A.; Hudson F., Acta Crystallogr.,1960,13: 660-667.
CrossRef - Ekwall P. Advances in Liquid Crsytals (G.H. Brown Ed.) Academic Press, N.Y., 1979.
- Friberg, S.E., Naturwissenschaften,1977,64: 612-618.
- Fontell, K. In Liquid crystal and plastic crystals, (P.A. Winsor and G.W. Gray Eds.), Ellis Horwood, Chichester, 1974.
- Friberg, S.E.;Osborne,D.W.,J Disp Sci. Technol.,1985,6(4): 485-495.
- Friberg S.E.; Hamdan,S.;Goldsmith L.; Rhein,L.,J Disp Sci. Technol. 1988, 9(4): 371-389.
- Friberg, S.E.; Goldsmith, L.; Hamdan, S.; Rhein, L., Colloids Surfaces. 1988, 30: 1-12.
CrossRef - Heuss, E., (1892). Monatshefte für praktische Dermatologie, 1892, 14: 400-409
- Schade, H.;Marchionini, A.,Klinische Wochenschrift,1928, 7: 12-14.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









