Removal of Chromium with The Complexing Agents from Industrial Effluents
J. L. Prameena Sheeja
Department of Chemistry, SRM University, Ramapuram.
Corresponding Author E-mail: prameenas@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/320452
Human activities and consequent developments have brought about the spectre of an overwhelming degradation of all facets of the natural environment-physical, chemical, biological and social. Environmental pollution, especially by chemicals, is one of the most significant factors in the degradation of the biosphere components. Among all chemical contaminants, heavy metals are believed to be of special ecological, biological and health significance. Unlike organic pollutants, the majority of which are susceptible to biological degradation, metal ions do not degrade into harmless end products. Chemical precipitation is a simple and economical method, and hence, has been widely used. The reduction of chromium (VI) to chromium (III) can be done with the help of ferrous sulphate. The precipitation was carried out in the presence and absence of complexing agents such as ammonium chloride, tartrate and citrate.
KEYWORDS:heavy metals; hydroxide precipitation; sulphide precipitation; complexing agents
Download this article as:| Copy the following to cite this article: Sheeja J. L. P. Removal of Chromium with The Complexing Agents from Industrial Effluents. Orient J Chem 2016;32(4). |
| Copy the following to cite this URL: Sheeja J. L. P. Removal of Chromium with The Complexing Agents from Industrial Effluents. Orient J Chem 2016;32(4). Available from: http://www.orientjchem.org/?p=18925 |
Introduction
Heavy metals are widely used in a variety of industrial activities and the wastes from these industries constitute a major source of heavy metal pollution in the environment. The heavy metals are toxic or poisonous at low concentrations. They cannot be degraded or destroyed. To a small extent they enter our bodies via food, drinking water and air. However, at higher concentrations they can lead to poisoning. Heavy metals are dangerous because they tend to bioaccumulate. One of the main causes of industrial pollution is the discharge of effluents containing heavy metals. Disposal of industrial wastewater has always been a major environmental issue. Pollutants in industrial wastewater are toxic that wastewater has to be treated before its reuse or disposal in water bodies. Since most of heavy metals are non-degradable into non-toxic end products, their concentrations must be reduced to acceptable levels before discharging them into environment. Otherwise these could pose threats to public health and affect the drinking water quality. Heavy metals can have serious effects on human and animal health. Beside the health effects, heavy metals are nonrenewable resources. Therefore, effective recovery of heavy metals is as important as their removal from waste streams.
Chemical treatment of industrial wastewater is preferable since industrial wastewaters are frequently complex, high in pollutant load and often containing materials toxic or resistant to the organisms on the biological processes. Chemical treatment systems are more predictable and inherently more subject to control by simple technique and chemicals are usually relatively tolerant to temperature changes.
Materials and Methods
All chemicals used were of Analytical Reagent (AR) grade. Double distilled water from an all glass still was used for preparing reagents. The instruments used for the study were pH meter (LI 120 ELICO make), high speed stirrer (model RW 16 B IKA Labortechnik), magnetic stirrer (model RH IKA Labortechnik) and Atomic Absorption Spectrophotometer (AAS) (Analytic Jena (AJ) Vario 6).
For hydroxide precipitation, pH of 200 mL of heavy metals solution (500 ppm) was raised from 7 to 11 using 1N NaOH. The contents were allowed to settle for 30 minutes. The supernatant was filtered through a Whatman No.42 filter paper and analysed using atomic absorption spectrophotometer for the presence of chromium. The precipitation was carried out in the presence and absence of complexing agents such as ammonium chloride, tartrate and citrate. The removal of chromium needed reduction of hexavalent form to trivalent form before precipitation as hydroxide. Ferrous sulphate was used as reducing agent at pH 2. In addition to pH, the ferrous sulphate dose was also optimized for chromium.
Results and Discussion
Experiments carried out for the removal of heavy metals using hydroxide precipitation at the optimum conditions at a reaction time of 5-60 minutes did not show any variation in the efficiency of chromium removal. Hence, 10 minutes was considered as the optimum reaction time.
Effect of ferrous sulphate dose
Hexavalent chromium can be reduced to trivalent form by ferrous ions by hydroxide precipitation. The effect of ferrous sulphate dose on the reduction of chromium was studied by varying from 0.2 g to 1g and keeping the pH at 2. The results are shown in Figure 1. The reduction increased with increase in ferrous sulphate dose and leveled off after a critical dose of 0.8g. At lower doses the reduction efficiency followed the order: absence of complexing agent>presence of ammonium chloride>tartrate>citrate.
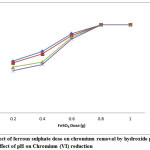 |
Figure 1: Effect of ferrous sulphate dose on chromium removal by hydroxide precipitation Effect of pH on Chromium (VI) reduction |
Effect of pH on Chromium (VI) reduction
The effect of pH on the reduction of chromium (VI) and eventually removal efficiency was studied in the pH range 1-6. The effect of reduction pH on the removal efficiency of chromium in the absence and presence of complexing agents are illustrated in Figure 2. It is observed that the removal efficiency was maximum at pH ≤ 2, and hence, it was taken as optimum. Chromium (VI) reduction is spontaneous at strongly acidic conditions, due to the instability of Chromium (VI) specified in acidic environment. The removal efficiency followed the order: absence of complexing agent>presence of ammonium chloride>tartrate>citrate.
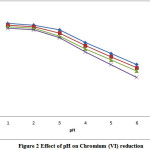 |
Figure 2: Effect of pH on Chromium (VI) reduction |
Effect of pH on Chemical precipitation
The effect of pH on the removal efficiency of chromium was studied in the pH range 7-11. The effect of pH on the removal efficiency of chromium in the absence and presence of complexing agents are illustrated in the Figure 3. It is observed that the removal efficiency increases with pH 8 and decreases thereafter. At high alkaline pH, chromium hydroxide gets converted to more soluble chromate. Hence, pH 8 was taken as optimum. The removal efficiency followed the order: absence of complexing agent>presence of ammonium chloride>tartrate>citrate.
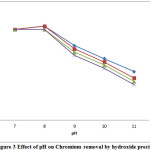 |
Figure 3: Effect of pH on Chromium removal by hydroxide precipitation |
Conclusion
Chemical precipitation method is used to achieve the superior metal removal required by the discharge limits. It removes the dissolved metal contaminants to the lowest levels possible while using the least amount of treatment chemicals and generating the least amount of sludge. It is a viable technology to remove complexed metals from wastewater. Chromium was removed as its hydroxide after reduction to chromium (III) using ferrous sulphate. The removal efficiency of chromium was found to be 99.85%.
References
- Barakat M.A, New trends in removing heavy metals from industrial wastewater, Arabian journal of chemistry, 2011, 4, 361-377.
CrossRef - Charles U. Pittman Jr, Dinesh Mohan, Activated carbons and low cost adsorbents for remediation of tri- and hexavalent chromium from water, Journal of Hazardous Materials, 2006, 137, 762-811.
CrossRef - Dinesh Mohan, Charles U. Pittman Jr, Arsenic removal from water/wastewater using adsorbents—A critical review, Journal of Hazardous Materials, 2007, 142, 1-53.
CrossRef - Fenglian Fu, Qi Wang, Removal of heavy metal ions from wastewaters: A review, Journal of Environmental management, 2011, 92, 407-418.
CrossRef - Hala Ahmed Hegazi, Removal of heavy metals from wastewater using agricultural and industrial wastes as adsorbents, HBRC Journal, 2013, 9, 276-282.
CrossRef - Hashim M.A, Soumyadeep Mukhopadhyay, Jaya Narayan Sahu, Bhaskar Sengupta, Remediation technologies for heavy metal contaminated groundwater, Journal of Environmental Management, 2011, 92, 2355-2388.
CrossRef - Mahmood M.BrbootI, Balasim A. AbiD and Najah M. Al-ShuwaikI, Removal of Heavy Metals Using Chemicals Precipitation, Eng.& Tech. Journal, 2011, 29, 595-612.
- Ming Hua, Shujuan Zhang, Bingcai Pan , Weiming Zhang, Lu Lv, Quanxing Zhang, Heavy metal removal from water/wastewater by nanosized metal oxides: A review, Journal of Hazardous Materials, 2012, 211, 317-331.
CrossRef - Mohammad Boshir Ahmed, John L. Zhou, Huu Hao Ngo, Wenshan Guo, Nikolaos S. Thomaidis, Jiang Xu, Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: A critical review, Journal of Hazardous Materials, 2006, 137, 762-811.
- Prameena Sheeja J.L., Physico-chemical characterization of pond water from Vaduvur, Mannargudi (tk), Thiruvarur district (India), Der Pharma Chemica, 2016, 8, 6-11.
- Prameena Sheeja, J.L., Assessment Of Macro And Micronutrients In Soils From Mannargudi Area, Thiruvarur District, Tamil Nadu, India, Research Journal of Chemical and Environmental Sciences Res J. Chem. Environ. Sci., 2015, 3, 32-37.
- Prameena Sheeja J.L., Advance technologies in the process of desalination, World Journal of Pharmacy and Pharmaceutical Sciences, 2015, 4, 875-886.
- Prameena Sheeja J.L., Selvapathy, P, Comparative study on the removal efficiency of cadmium and lead using hydroxide and sulfide precipitation with the complexing agents, International Journal of Current Research in Chemistry and Pharmaceutical Sciences, 2014, 1, 38-42.
- Omar E. Abdel Salam, Neama A. Reiad, Maha M. ElShafei, A study of the removal characteristics of heavy metals from wastewater by low-cost adsorbents, Journal of Advanced Research, 2011, 2, 297-303.
CrossRef - Thomas S.Y. Choong, T.G. Chuah, Y. Robiah, F.L. Gregory Koay , I. Azni, Arsenic toxicity, health hazards and removal techniques from water: an overview, Desalination, 2007, 1, 139-166.

This work is licensed under a Creative Commons Attribution 4.0 International License.









