Recent Advancements and Biological Activities of Aryl Propionic Acid Derivatives: (A Review)
Harshita Dhall, Pratiksha Sikka, Arvind Kumar and Arun K. Mishra
Central Facility of Instrumentation, Faculty of Pharmacy, IFTM University, Moradabad-244001, India.
Corresponding Author E-mail: harshitadhall03@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/320411
Article Received on :
Article Accepted on :
Article Published : 03 Aug 2016
The aryl propionic acid derivatives belong to an important class of NSAIDs (Non Steroidal Anti-inflammatory Drugs). Ibuprofen, chemically called 2-(4-isobutyl phenyl) propionic acid, is a well known NSAID. Aryl propionic acid derivatives possesses a wide range of biological activities including anti-bacterial, anti-convulsant, anti-cancer, analgesic and anti-inflammatory activities. Apart from very potent compounds in the field of analgesics and antipyrectics as Ibuprofen, Oxaprozin, Ketoprofen, Fenoprofen; aryl propionic acid derivatives plays important role to treat other ailments also. Through this review, an attempt has been made to emphasize on recent work done and recent advancements in arena of aryl propionic acid derivatives in view of medicinal chemistry.
KEYWORDS:Analgesic activity; Anti-bacterial activity; Anti-cancer activity; Anti-convulsant activity; Aryl propionic acid
Download this article as:| Copy the following to cite this article: Dhall H, Sikka P, Kumar A, Mishra A. K. Recent Advancements and Biological Activities of Aryl Propionic Acid Derivatives: A Review. Orient J Chem 2016;32(4). |
| Copy the following to cite this URL: Dhall H, Sikka P, Kumar A, Mishra A. K. Recent Advancements and Biological Activities of Aryl Propionic Acid Derivatives: A Review. Orient J Chem 2016;32(4). Available from: http://www.orientjchem.org/?p=19834 |
Introduction
Medicinal chemistry is the discipline which is concerned in determining the influence of chemical structure on biological activity. Medicinal chemistry developed from an empirical one involving organic synthesis of novel compounds is based largely on the modification of structure and identification of their biological activity.Medicinal chemistry also involves the discovery, development, interpretation and the identification of mechanism of action of biologically active compounds at the molecular level1.
The aryl propionic acid derivatives are a wide and important family of Non steroidal anti-inflammatory drugs (NSAIDs) 2. NSAIDs are widely used for the treatment of different types of arthritis and musculoskeletal disorders3-8. The biological response of NSAIDs results from the suppression of prostaglandin (PG) biosynthesis in which the cyclooxygenase (COX) enzyme is the key intermediate in the biosynthesis of prostaglandin from arachidonic acid9, 10. It was discovered during early 1990s, that COX exists in two isoforms, namely constitutive COX-1, which provides cytoprotection in the gastro-intestinal (GI) and the other inducible COX-2 which mediated inflammation11-13.
One of the NSAIDs viz. Ibuprofen, chemically called 2-(4-isobutyl phenyl) propionic acid, is a well known analgesic which possess potent pain relieving, anti pyretic and anti-inflammatory properties14,15. It is particularly known for its use in pain relief from arthritis16. Long term use of NSAIDs results in gastro-intestinal ulceration, bleeding and nephrotoxicity17. The gastro-intestinal damage is generally associated with two factors which include local irritation by carboxylic acid moiety, which is common in most NSAIDs (topical effect) and decreased production of tissue prostaglandin (PGs), which minimizes the physiological role of cytoprotective prostaglandins in maintaining GI health and homeostasis18. Studies showed that forming the deriviative of the carboxylate function of representative NSAIDs resulted in an enhanced anti-inflammatory activity with reduced ulcerogenic effect. Moreover, certain compounds bearing 1, 3, 4-oxadiazole/thiadiazole and 1, 2, 4-triazole nucleus have been reported to bear significant anti-inflammatory activity19, 20. In the last two decades, there has been a considerable amount of work in the role of reactive oxygen species in inflammation. Inflammation is one of the manifestations of oxidative stress and the pathways that generate the mediators of inflammation such as adhesion molecules and interleukins21.
Pharmacological Activities
Anti-Bacterial activity
Singh S. A. and Bhat S. V. synthesised twenty Baylis-Hillman adducts (3-hydroxy-2-methylene-3-phenylpropionic acid derivatives) from different aromatic aldehydes and activated vinyl derivatives. These were screened for their anti microbial activity in vitro by serial dilution method. The synthesized compounds (1-15) showed potent anti bacterial activity (Table 1)22.
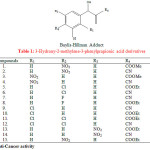 |
Table 1: 3-Hydroxy-2-methylene-3-phenylpropionic acid derivatives |
Anti-Cancer Activity
Rayam P. et al synthesised a series of acyl hydrazone derivatives of 3-(4, 5-diphenyl-1, 3-oxazole-2-yl) propionic acid. The intermediate N- acyl hydrazine was prepared from NSAID oxaprozin, was coupled with a variety of aromatic aldehydes under conventional as well as microwave irradiation conditions. The synthesised compounds (Table 2) were further screened for in vitro anti cancer activity. Compounds (1-7) showed potent anticancer activity when the comparison was made with Cisplatin23.
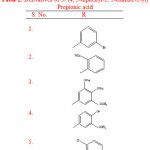 |
Table 2: Derivatives of 3- (4, 5-diphenyl-1, 3-oxazole-2-yl) Propionic acid |
Anti-Convulsant activity
Semwal A. synthesised a series of β, β- diphenyl propionic acid amides which were evaluated for anti-convulsant activity using maximal electroshock seizures (MES) method. The compounds (1-8) showed mild to moderate anti-convulsant activity ranging from 0.0% to 50% (Table 3). Indomethacin was taken as standard drug24.
Table 3: β, β-Diphenyl Propionic acid amides
| S. No. | R |
| 1. | H |
| 2. | C6H4 |
| 3. | C6H4 |
| 4. | p.(CH3O)C6H4 |
| 5. | 2-C5H4N |
| 6. | 2COOCH3C6H4 |
| 7. | SO2NH2C6H4 |
| 8. | -NH-CH2-COOH |
Dogruer D. S. et al synthesised sixteen new amide derivatives by treatment of 2-[5, 6-diphenyl-3(2H)-pyridazinone-2-yl] acetic acid or 3-[5, 6-diphenyl-3(2H)-pyridazinone-2-yl] propionic acid with appropriate amine derivatives in the presence of triethylamine and ethyl chloroformate in dichloromethane at room temperature (Table 4). Out of which, Compounds (1-4) showed higher analgesic activity. Aspirin was taken as standard drug25.
Analgesic Activity
![Table 4: 2-[5, 6-diphenyl-3(2H)-pyridazinone-2-yl] acetic acid derivatives](http://www.orientjchem.org/wp-content/uploads/2016/08/Vol32No4_Rece_Hars_table4-150x150.jpg) |
Table 4: 2-[5, 6-diphenyl-3(2H)-pyridazinone-2-yl] acetic acid derivatives |
Rayam P. et al synthesised a series of acyl hydrazone derivatives of 3-(4, 5-diphenyl-1, 3-oxazole-2-yl) propionic acid. The key intermediate N- acyl hydrazine was prepared in good yield from NSAID oxaprozin, was coupled with a variety of aromatic aldehydes under conventional as well as microwave irradiation conditions. The compounds were screened for in vivo analgesic activity and compounds (1-3) exhibited significant in vivo analgesic activity (Table 5). Oxaprozin was taken as reference drug23.
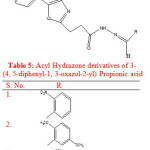 |
Table 5: Acyl Hydrazone derivatives of 3-(4, 5-diphenyl-1, 3-oxazol-2-yl) Propionic acid |
Anti-inflammatory Activity
Semwal A. synthesised a series of β, β- diphenyl propionic acid amides. The synthesised compounds were evaluated for its anti inflammatory activity by carrageenan induced paw edema method. The compounds (1-8) showed mild to moderate anti inflammatory activity ranging from 36.13% to 90% after 3 hrs whereas the standard drug indomethacin showed 81.25% inhibition (Table 6) 24.
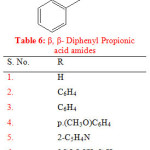 |
Table 6: β, β- Diphenyl Propionic acid amides |
Dogruer D. S. et al synthesised sixteen new amide derivatives (Table 7) by treatment of 2-[5,6-diphenyl-3(2H)-pyridazinone-2-yl] acetic acid or 3-[5,6-diphenyl-3(2H)-pyridazinone-2-yl] propanoic acid with appropriate amine derivatives in the presence of triethylamine and ethyl chloroformate in dichloromethane at room temperature. Out of which, compounds (1-3) showed potent anti inflammatory activity25.
![Table 7: 2-[5, 6-diphenyl-3(2H)-pyridazinone-2-yl] acetic acid derivatives](http://www.orientjchem.org/wp-content/uploads/2016/08/Vol32No4_Rece_Hars_table7-150x150.jpg) |
Table 7: 2-[5, 6-diphenyl-3(2H)-pyridazinone-2-yl] acetic acid derivatives |
Rayam P. et al synthesised a series of acyl hydrazone derivatives of 3-(4, 5-diphenyl-1, 3-oxazole-2-yl) propionic acid. The key intermediate N- acyl hydrazine was prepared in good yield from NSAID oxaprozin, was coupled with a variety of aromatic aldehydes under conventional as well as microwave irradiation conditions. The synthesised compounds (Table 8) were screened for in vivo anti inflammatory activity and compounds (1-3) exhibited significant in vivo anti inflammatory activity. Oxaprozin was taken as reference compound23.
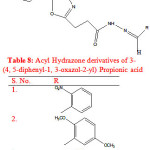 |
Table 8: Acyl Hydrazone derivatives of 3-(4, 5-diphenyl-1, 3-oxazol-2-yl) Propionic acid |
Dilber S. P. et al synthesized β-hydroxy-β-aryl propanoic acids by a two-step process. The first step involves the synthesis of diastereomeric 3-hydroxy-2-methyl-3-(4-biphenylyl) butanoic acids whereas the second step was a modified Reformatsky reaction in presence of Zn in tetrahydrofuran (THF) at -5 to 10oC between synthesized compound of first step and 4-acetylbiphenyl. The synthesized compounds were screened for anti-inflammatory activity and only two compounds (1, 2) showed strongest anti-inflammatory activity26.
Gupta R. et al synthesized 2-(4-sec-butyl-phenyl)-propionic acid-pyrrolidin-2-yl-carbamoyl methyl esters by refluxing ibuprofen with 2-aminopyridine in chloroacetyl chloride in presence of glacial acetic acid. The synthesized compounds were evaluated for anti-inflammatory activity. Compounds (1-6) showed potent anti-inflammatory activity (Table 9) 27. 
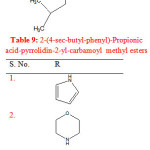 |
Table 9: 2-(4-sec-butyl-phenyl)-Propionic acid-pyrrolidin-2-yl-carbamoyl methyl esters Click here to View table |
Recent Advancements In Area Of Propionic Acid Derivatives
Studies on structure activity relationship (SAR) were conducted on 3-arylpropionic acidsas selective agonists by introducing substituents to the chain of propionic acid the adjacent phenyl ring with pyridine chain was replaced to develop a series of containing modified 3-arylpropionic acids with enhanced half-life in rat28. The important members of this class, ibuprofen, naproxen and ketoprofen are now available as OTC medicines. However, the indiscriminate use of these drugs without a physician’s prescription has resulted in an increased incidence of acute and chronic renal failure in adolescents. All compounds belonging to aryl and heteroarylpropionic acids (except oxaprozin) possess a chiral carbon in the α-position of the acetic acid side chain. Although most of the compounds are marketed as racemates, only the (S)-enantiomer was found to have any inhibitory activity against the COX isoenzymes. Therefore, only (S)-enantiomer is believed to be responsible for the observed therapeutic action as well as the drug-induced GI side effects and nephrotoxicity29. The details of some potent compounds and their pharmacological activities associated are presented in Table 10.
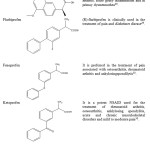 |
Table 10: Potent Compounds and their Pharmacological Significance |
Conclusion
On the basis of various literature surveys, aryl propionic acid derivatives show various activities such as anti bacterial, anti cancer, anti convulsant, analgesic and anti inflammatory. The possible improvements in the activity can be further achieved by slight modifications in the substituents on the basic aryl propionic acid.
References
- Vadi, M.; Omidi, A. Oriental J. Chem. 2012, 28, (3), 1325-1330.
- Melnikova, I. Nat. Rev. Drug. Discov. 2005, 4, 453-454.
CrossRef - Tayseer, A.; Manal, A.D.; Hamdi, M.H. Molecules. 2002, 7, 494-500.
CrossRef - Cansiz, A.; Koparir, M.; Demirdag, A. Molecules. 2004, 9, 204-212.
CrossRef - Katica, C.; Vesna, D.; Vlado, K.; Dora, G.M.; Aloksandra, B. Molecules. 2001, 6, 815-824.
CrossRef - Li-Xue, Z.; An-Jian, Z.; Xian-Xin, C.; Xin-Xiang, Xiang-Yun, N.; Dong-Yung, C.; Zi-Yi, C. Molecules. 2002, 7, 681-689.
- Hovsepjian, T.R.; Dilanian, E.R.; Enmgoyan, A.P.; Melikohanjaian, R.G. Chem. Het. Compd. 2004, 40, (9), 1194-1198.
CrossRef - Wasfy, A.A.F. J. Chem. Res. 2003, 8, 457-458.
CrossRef - Smith, C.J.; Zhang, Y.; Koboldt, C.M. Proc. Natt. Acad. Sci. 1998, 95, 1313-1318.
CrossRef - Warner, T.D.; Giuliani, F.; Vaynovie, I.; Bukasa, A.; Mitchell, J.A.; Vave, J.R. Proc. Acad. Sci. 1999, 96, 7563-7568.
CrossRef - Khan, S.A.; Imran, M.; Arshad, M.F.; Kumar. S. Oriental J. Chem. 2004, 20, 2, 265-269.
- Dannharat, G.; Kiefer, W. Eur. J. Med. Chem. 2001, 36, 109-126.
CrossRef - Marnett, L.J.; Kalgutkar, A.S. Curr. Opin. Chem. Biol. 1998, 2, 482-490.
CrossRef - Jayashankar, B.; Lokanathrai, K.M.; Baskaran, N.; Sathish, H.S. Eur J. Med. Chem. 2009, 44, 3898-3902.
CrossRef - Manjunatha, K.; Poojary, B.; Lobo, L.P.; Fernandes, J.; Suchethakumari, N. Eur. J. Med. Chem. 2010, 45, 5225-5233.
CrossRef - Zoung, H.L.; Jin, H.W.; Seong, J.C.; Jong, D.J.; Gwan, G.S. Rheumatol Int. 2010, 30, 357-363.
CrossRef - Amir, M.; Kumar, S. Acta Pharm. 2007, 57, 31-45.
CrossRef - Amir, M.; Kumar, S. Arch. Pharm. Chem. Life. Sci. 2005, 338, 24-31.
- Ali, K.F. N. Oriental J. Chem. 2015, 31,1, 239-247.
- Duflos, M.; Mourrisson, M.R.; Brolet, J. Eur. J. Med. Chem. 2001, 36, 545-553.
CrossRef - Khausari, N.; Shakiba, Y.; Mahmaoudi, M. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 73-80.
CrossRef - Singh, S.A.; Bhat, S.V. Acta Pharm. 2011, 61, 447-455.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









