Preparation and Characterization of Amorphous Silica and Calcium Oxide from Agricultural Wastes
Supachai Sompech1,Thananchai Dasri1 and Sukhontip Thaomola2,*
1Faculty of Applied Science and Engineering, NongKhai Campus, KhonKaen University, NongKhai, 43000, Thailand.
2Faculty of Science and Arts, Chanthaburi Campus, Burapha University, Chanthaburi, 22170, Thailand.
Corresponding Author E-mail: suky_2547@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/320418
Article Received on :
Article Accepted on :
Article Published : 06 Aug 2016
Rice husk ash and bagasse ash were agricultural wastes that provide an abundanceof the silica (SiO2) source and the chicken eggshells and duck eggshells were important sources forcalcium oxide (CaO). Therefore, in this study the rice husk ash and bagasse ash were used as raw materials for synthesisofsilica powder,while chicken eggshells and duck eggshells were synthesized forthe calcium oxide.The results from the XRD pattern clearly showedthe structural formation of amorphous SiO2 and CaO phase. While the FTIR results indicated that the spectrums which displayedthe characteristic peaks of the functional groups presenting in the SiO2 and CaOpowder. However, the SEM images revealed that the particles agglomerated, various sizes and the particle size were found to be in micron level.
KEYWORDS:Rice husk ash; Bagasse ash; Chicken eggshells; Duck eggshells; Agricultural wastes
Download this article as:| Copy the following to cite this article: Sompech S, Dasri T, Thaomola S. Preparation and Characterization of Amorphous Silica and Calcium Oxide from Agricultural Wastes. Orient J Chem 2016;32(4). |
| Copy the following to cite this URL: Sompech S, Dasri T, Thaomola S. Preparation and Characterization of Amorphous Silica and Calcium Oxide from Agricultural Wastes. Orient J Chem 2016;32(4). Available from: http://www.orientjchem.org/?p=20287 |
Introduction
Rice husk and bagasse areagricultural waste material abundantly available in Thailand.They areimportant biomass sources, which used as a fuel in the brick industry and sugar industry, respectively. As a result, a large quantity of rice husk ash and bagasse ash are generated and created a serious dispensation problem.Rice husk ash and bagasse ash are rich in silica (SiO2)(more than 60% for bagasse ash and above 90% for rice husk ash) and can be used as a raw material for produce the silica powderproduction1-5.Many studies have reported thatthe rice husk ash and bagasse ash were an excellent sources for synthesis of amorphous silica6-9 becausethe fine silica powder is a basic raw material that is widely usedin electronic substrates, thermal and electrical insulators, optoelectronic devices and composite fillers, etc.10.
While the chicken eggshells and duck eggshells are one group of agricultural waste materials which can be found as waste products from restaurants and fresh markets in Thailand, as they areused for cooking. The chemical composition of eggshells is calcium carbonate (CaCO3)typically containing a high level (above 90%)11, 12.As the chicken and duck eggshells are composed of calcium carbonate, so being calcinedat above 700 °Cwill produce the CaO powder13, 14, which can be used as a basic adsorbent for carbondioxide (CO2)15 and as a catalyst for the production ofbiodiesel13.
Therefore, the aim of this study was to synthesize of amorphous silicaand calcium oxide powder from agricultural wastes by using the rice husk ash and bagasse ash used as sources for silica, while the chicken eggshells and duckeggshells were sourcesfor calcium oxide. The synthesized powder were characterized by Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD),Brunauer-Emmett-Teller (BET), particle size analyzerand scanning electron microscopy (SEM).
Materials and Methods
Synthesis of Sample
Rice husk ashand bagasse ash were obtained from a brick factory and sugar industry, respectively and theywere rinsed by water several times to removeimpurity and then burned at 700°C for 3 h. To evaluate the chemical compositions of the rice husk ash and bagasse ashwas analyzed by X-ray fluorescence (XRF), this resultwas shown in Table 1.Subsequently, twenty grams of rice husk ash and bagasse ash sampleswere stirred in 80 ml of 2.5 N sodium hydroxide solutions, afterwards heated in a covered 250 ml Erlenmeyer flask for 3 h. After that, the solution was filtered and the residue was washed with 40 ml of boiling water. The filtrate was allowed to cool down at room temperature. Then, it was added by 5 N H2SO4 with constant stirring until pH 2. Then,the filtrate was added NH4OH until pH 8.5 left for 3 h and dried at 100°C for 12 h and finally waspulverized by mortar.
Simultaneously, the eggshells waste received from restaurant were rinsed with water several times to remove additional residues or any unwantedsubstancefromthe surface and then heated at 100oC for 12 h to dry by oven. Completely dried eggshells was pulverized by using a hammer mill, after that crushed with a juice blender and then passed through a sieve.Next the chemical compositions of eggshells were analyzed by X-ray fluorescence (XRF) shown in Table 2. The dried eggshells werecalcined in a muffle furnace under static air conditions at 900oC for 3 h to transform the calcium carbonate toCaO powder.
Characterization
The powders obtained were characterized by various techniques. The average particle size distribution was determined by using particle size analyzer (Mastersizer S, Malvern) and examination the functional groups studied by Fourier transforms infrared spectrometer (Perkin Elmer spectrometer). X-ray diffractometer(Phillips X’ pert X-ray diffractometer) was used to identify phase of the powder sample. The specific surface area of the powder sample was measured using the BET nitrogen adsorption method (Quantachrome), and morphologyof sample observed by scanning electron microscope (LEO/1450).
Results and Discussion
The chemical composition of the raw materials for this study is shown in Table 1 and Table 2.In the Table 1, it showedthat the chemical composition of rice husk ash and bagasse ash samples, after burning out at 700oC for 3 h,presented the highest amount of SiO2; while,the major component of both eggshells displayedcalcium CaCO3 (Table 2).
Table 1: Chemical composition of rice husk ash and bagasse ash
| Samples |
Inorganic component (wt.%) |
||||||||||
|
SiO2 |
Al2O3 |
MgO |
Fe2O3 |
CaO |
P2O5 |
Mn2O3 |
SO3 |
K2O |
TiO2 |
Total |
|
| Rice husk ash |
93.57 |
0.80 |
0.66 |
0.59 |
0.90 |
1.02 |
0.28 |
0.10 |
2.01 |
0.08 |
100 |
| Bagasse ash |
80.46 |
1.24 |
4.07 |
1.14 |
4.43 |
5.24 |
0.25 |
0.69 |
2.36 |
0.15 |
100 |
Table 2: Chemical composition of egg shells
| Samples |
Inorganic component (wt.%) |
||||||||||
|
CaCO3 |
Al2O3 |
MgO |
Fe2O3 |
SiO2 |
P2O5 |
Mn2O3 |
SO3 |
K2O |
TiO2 |
Total |
|
| Chicken egg |
99.66 |
– |
– |
– |
– |
– |
– |
0.34 |
– |
– |
100 |
| Duck egg |
99.19 |
– |
– |
– |
– |
– |
– |
0.82 |
– |
– |
100 |
The X-ray diffraction pattern of SiO2 powderobtained from rice husk ash and bagasse ash is shown in Fig.1. The results showed the strong broad peakataround22º (2θ) of both sample after calcining ash at 700°Cfor 3 h. This resultsconfirmedthat this peak is nature of silica, which supposed to be the characteristic of amorphousSiO2. For the results from FT- infrared (FTIR) spectrum of SiO2 powder is presented in Fig. 2. It clearly showed aslightly broad band since 2,800 to 3,700 cm-1which assigned to present the OH stretching frequency of silanol groups and adsorbed water. A strong intense band at 1,100 cm-1is associated to the siloxane Si-O-Si vibration of the molecules.Amorphous silica exhibits a relatively strong peak around at 800 cm-1.
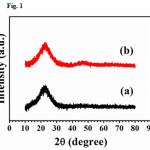 |
Figure 1: XRD patterns of SiO2 powder after prepared from (a) rice husk ash and (b) bagasse ash Click here to View Figure |
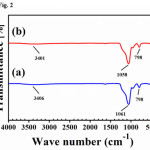 |
Figure 2: FTIR spectrum of SiO2 powder after prepared from (a) rice husk ash and (b) bagasse ash Click here to View Figure |
For the X-ray diffraction pattern of the CaO powder obtained from chicken eggshellsand duck eggshells are shown in Figs. 3 and 4, respectively. The results revealed that the thermal process of CaCO3 phase is completely decarbonized and turned to beCaOphaseafter both chicken and duck eggshells werecalcined at 900oC.The shape of the sharp and strong diffraction peaks indicated that the samples are well crystallized andcorresponded to the standard values of file JCPDS No. 37-1497.
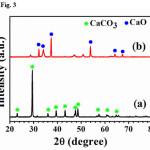 |
Figure 3: XRD pattern of chicken eggshells (a) beforecalcined and (b) after calcined at 900 oC Click here to View Figure |
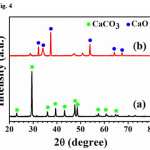 |
Figure 4: XRD pattern of duck eggshells (a) beforecalcined and (b) after calcined at 900 oC Click here to View Figure |
Fig. 5 showed the FT-infrared (FTIR) spectrum of the CaOpowder after prepared from chicken eggshells and duck eggshells, respectively. In Fig. 5a and 5b presented the strong band at 3638 cm-1can be attributed to the (OH) stretching vibration. The broad band around 1400 cm-1indicated the symmetric stretching vibration of carbonate. Small band at 874 cm-1 demonstrated the presence of carbonate species. The tiny band at550 cm-1 identified vibration of the Ca-O bond16.
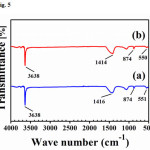 |
Figure 5: FTIR spectrum of CaO powder after prepared from (a) chicken eggshells and (b) duck eggshells Click here to View Figure |
While, the average particle size and specific surface area of the SiO2 and CaO powder is shown in Table 3.The results displayed the average particle size of the amorphous SiO2 powderobtained from rice husk ash and bagasse ashis not significantly different, the particle sizes are 46.74 µm and 47.16 µm, respectively. In addition, the specific surface area ofamorphous SiO2powder was also similar as well. However,the average particle size of the CaO powderobtained from chicken and duck eggshells after being calcined of CaCO3phase are 57.13 µm and 23.91 µm, respectively. And the specific surface area of CaOpowderare 13.20 m2/g and 16.42 m2/g, respectively.
Table 3: Average particle size and specific surface area of prepared samples
| Samples |
Average particle size [D 4,3] (µm) |
Specific surface area (m2/g) |
| SiO2 from rice husk ash |
46.74 |
271.21 |
| SiO2 from bagasse ash |
47.16 |
233.85 |
| CaO from chicken eggshells |
57.13 |
13.20 |
| CaO from duck eggshells |
23.91 |
16.42 |
The morphology of the amorphous SiO2 powderobtained from rice husk ash and bagasse ash is shown in Fig. 6. The SEM images indicated that the particles of powder areagglomerated, various sizes, irregular morphology, and particles size more than 1 μm were recognized. In addition, the average particle size of SiO2 powder obtained from rice husk ash is in the range of 1-7 μm (Fig. 6a), while theaverage particle size of SiO2 powder obtained from bagasse ash is in the range of 1-10 μm (Fig. 6b).
 |
Figure 6: SEM micrographs of amorphous SiO2 powder after prepared from (a) rice husk ash and (b) bagasse ash Click here to View Figure |
Fig. 7 showed the SEM images of the CaOpowder aftercalciningof CaCO3 fromchicken eggshells and duck eggshells. In Fig. 7a can be seen that the average particle size and shape of CaOparticles obtained from chicken eggshells wassimilar to theCaO particles obtained from duck eggshells as shown in Fig. 7b. The characteristic of CaO particles of both chicken eggshells and duck eggshellsare regular micro morphology of rod like, the average particles with size ranging from 2µm to 4µm of width are observed.
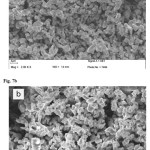 |
Figure 7: SEM micrographs of CaO powder after prepared from (a) chicken eggshells and (b) duck egg shells Click here to View Figure |
Conclusion
Amorphous SiO2is successfully synthesized from the rice husk ash and bagasse ash thatused asraw materials. While the CaO powder can be synthesized by using CaCO3from chicken eggshells and duck eggshells thatused as raw materials, and thenburning at 900oC for 3 h.The XRD results clearly exhibited the structural formation of SiO2 and CaO phase. The FTIR spectrums demonstrated that the characteristic peaks of the important functional groups present in the powder samples of SiO2 and CaO. The SEM images illustrated that the particles are agglomerated, various sizes, and the particle size is found to be in the micro regime.This study revealed that the agricultural waste material of rice husk ash, bagasse ash, chicken eggshells, and duck eggshells acts as an alternative source for theproduction of SiO2 and CaO powder.
Acknowledgments
This work was financial supported by the young researcher development project of KhonKaen University.
References
- Thuadaij, N.;Nuntiya, A.Chiang Mai J. Sci.2008, 35, 206-211.
- Yalçin, N.;Sevinç, V.Ceram. Int. 2001, 27, 219-224.
CrossRef - Thuadaij, N.;Nuntiya, A. CMU J. Nat. Sci.2008, 7, 59-65.
- Hariharan, V.;Sivakumar, G. Int. J. Chem. Tech. Res.2013, 5, 1263-1266.
- Nazriati, N.;Setyawan,H.; Affandi, S.;Yuwana, M.;Winardi, S.J. Non-Cryst. Solids, 2014, 400, 6-11.
CrossRef - Real, C.; Alcala, M.D.;Criado, J.M.J. Am. Ceram. Soc.1996, 79, 2012-2016.
CrossRef - Nandi, K. C.;Biswas, A. K.; Acharya, H. N.Mater.Lett. 1991, 12, 171-174.
CrossRef - Faria, K. C. P.;Gurgel, R. F.;Holanda, J. N. F. J. Environ. Manage. 2012, 101, 7-12.
CrossRef - Aigbodin, V.S.; Hassan, S. B.;Ause, T.;Nyior, G. B.J. Mineral Mater.Charac. Eng. 2010, 9, 67-77.
- Liou, T.-H.Mater. Sci. Eng. A,2004, 364, 313-323.
CrossRef - Ok, Y. S.; Lee, S. S.;Jeon, W.-T.; Oh, S.-E.; Usman, A. R. A.; Moon, D. H.Environ. Geochem. Health, 2011, 33, 31-39.
CrossRef - Cho, Y. B.;Seo, G.Bioresour. Technol.2010, 101, 8515-8519.
CrossRef - Wei, Z.;Xu, C.; Li, B.Bioresour. Technol. 2009, 100, 2883-2885.
CrossRef - Niju, S.;Meera, K. M.; Begum, S.;Anantharaman, N.J. Saudi. Chem. Soc. 2014, 18, 702-706.
CrossRef - Ives, M.; Mundy, R. C.; Fennell, P. S.; Davidson, J. F.; Dennis, J. S.;Hayhurst, A. N. Energ. Fuels, 2008, 22, 3852-3857.
CrossRef - Mirghiasi, Z.; Bakhtiari, F.; Darezereshki, E.; Esmaeilzadeh, E. J. Ind. Eng. Chem. 2014, 20, 113-117
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









