Electrochemical and Corrosion Inhibition Studies of Cucurbita Maxima
K. Anbarasi
Nirmala College for Women, Coimbatore-641018, India.
Corresponding Author E-mail: anbarasi12@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/320443
The influence of the acid extract of peel of Cucurbita maxima (PCM) on the corrosion of mild steel in 1N H2SO4 was investigated by weight loss, polarization and impedance methods and SEM analysis. The inhibition efficiency increases with extract concentration and immersion period. Weight loss and corrosion rates of mild steel decreased as the concentration of inhibitor increased. The results showed that PCM was potential corrosion inhibitor and maximum inhibition efficiency (IE %) obtained was 98% for 3%PCM at 1h. Impedance measurement results an increase in charge transfer resistance (Rct), which also confirms the corrosion inhibitive nature of the plant extract. Potentiodynamic study showed that PCM acts as a mixed type of inhibitor, which controls both the anodic and cathodic reactions. Scanning electron microscopic studies provided the evidence of improved surface condition for the corrosion protection, due to the adsorption.
KEYWORDS:Adsorption; Polarization; Impedance; Corrosion rate; Inhibition efficiency
Download this article as:| Copy the following to cite this article: Anbarasi K. Electrochemical and Corrosion Inhibition Studies of Cucurbita Maxima. Orient J Chem 2016;32(4). |
| Copy the following to cite this URL: Anbarasi K. Electrochemical and Corrosion Inhibition Studies of Cucurbita Maxima. Orient J Chem 2016;32(4). Available from: http://www.orientjchem.org/?p=18959 |
Introduction
Acid solutions are widely used such as in acid pickling, industrial cleaning, oil well cleaning, etc [1].Corrosion inhibitors are used to reduce the corrosion rate of metals in contact with acidic environment [2].Most of the inhibitors are organic compounds with hetero-atoms having higher electron density, are adsorbed on the metallic surface and block the active sites of the metal atom. They are highly toxic to environment. Hence, use of natural plant extracts as eco-friendly and biodegradable corrosion inhibitors has become popular. Literature study shows that green corrosion inhibitor with no environmental hazards is to be discovered or invented[3].On this note Natural products such as Nyctanthes arbortristis[4], Acacia trees[5],Aloes extract[6], Terminalia catappa [7], Cordia Dichotoma [8], Rauvolfia serpentine[9], Ginkgo leaves[10] Garlic aqueous extract[11], Green leafy vegetables[12] and Bauhinia purpurea[13] have been used as inhibitors. Cucurbita maxima belongs to the family cucurbitaceae. Cucurbita species is found to contain flavonoids, alkaloids, carotenoids, steroids, saponins, carbohydrates and amino acids [14].
In the present work investigates the corrosion inhibition properties of the acid extract of the peel of Cucurbita maxima (PCM) on mild steel by weight loss measurement, polarization and impedance methods and SEM analysis.
Experimental
Materials
The sheet of mild steel (MS) used for this study has the following composition (wt %) (0.047% C; 0.22% Mn; 0.015% Ni; 0.001% S; 0.006% Al;0.002% p;0.001% Mo; Fe balance).The sheet was 2 mm in thickness and was mechanically press cut into 5 cm 2 cm coupons. The 1N H2SO4 Solution, prepared from AR grade H2SO4 was employed as the corrodent for the study. The extract was prepared by refluxing 25g of powdered peel of Cucurbita maxima in 500 ml of 1N H2SO4 for 3 h. and kept overnight. Then it was filtered and this was taken as a stock solution. An inhibitor test solution was prepared in the concentration range from 0.05% v/v to 3% v/v from the respective stock solution.
Methods
Weight loss method
The Weight Loss method was the best known and simplest corrosion monitoring techniques. The mild steel specimens were abraded with a series of emery papers (grade 400-600), washed with distilled water, degreased with acetone, and dried. After weighing accurately on an analytical balance, three parallel MS specimens were completely immersed in a beaker containing 100 ml of 1N H2SO4 with and without different concentrations of PCM at different immersion periods (1h, 3h, 5h, 7h, 18h and 24h). Then, the mild steel specimens were removed, washed with distilled water, dried and weighed accurately. The experiment was carried out in triplicate and the weight losses were averaged. From the weight loss measurements, the corrosion rate (CR) and Inhibition efficiency (IE) will be calculated using the following relationship [15]
CR (mpy) = 534 W/ D A t
Where W is the weight loss in mg, D is the density of mild steel (7.8/cm3), A is the area of specimen (cm2) and t is the time of immersion
IE %= [CRblank – CRinhi / CRblank] 100
Where CRblank and CRinhi are the corrosion rate values in absence and in presence of inhibitor.
Potentiodynamic polarization
Electrochemical measurement providing vital information about the mechanism of inhibition. The measurement was carried out using IVIUM make analyzer at room temperature with and without the addition of different PCM concentrations at a scan rate of 1 mV/s. The potential range was calculated from Eocp values. The inhibition efficiency was calculated by Tafel method and LPR method .The calculation of IE from Tafel and LPR methods are given by the following equation [16]
IE % = [ (Icorr(blank) – Icorr(inhibitor)) / Icorr(blank) ] × 100
IE % = [(Rp(inhibitor) – Rp(blank)) / Rp(inhibitor)] × 100
Where Icorr(blank) and Icorr(inhibitor) are referred to corrosion current density in the absence and presence of PCM respectively. Rp(blank) and Rp(inhibitor) are referred to resistance polarization without and with the addition of PCM respectively.
Electrochemical Impedance Spectroscopy (EIS)
Eocp of every sample was immersed for 30 minutes over a frequency range of 10 KHz to 0.01 Hz with signal amplitude of 0.025 V and scan rate of 1 mV/s. The inhibition efficiency was calculated from Rct and cdl values are given by the following equations [17].
IE % = [( Rctinhibitor – Rctblank) / Rctinhibitor ] × 100
Where Rctblank and Rctinhibitor are referred to charge transfer resistance without and with the addition of PCM extract respectively.
Scanning Electron Microscopy (SEM)
The mild steel specimens immersed in blank and in the inhibitor solutions for 3h were removed, rinsed with distilled water, dried and examined using Shimadzu make scanning electron microscope in the magnification range of 100 μm.
Results and Discussions
Effect of immersion time on inhibition efficiency of the plant extract in IN H2SO4 medium
The inhibition efficiency of the plant extract and corrosion rate of mild steel for various immersion times was shown in Table1. Corrosion rate of mild steel decreases and inhibition efficiency increases with the increased inhibitor concentration. Figure 1 shows the effect of immersion periods on the corrosion rate of mild steel and Figure 2 shows the relation between the inhibition efficiency and different immersion periods. At 3% v/v of the inhibitor concentration for 1 hour the IE was found to be 98%. The decrease in corrosion rate and increase in inhibitor efficiency usually attributed to the adsorption of plant constituents on the surface of mild steel which makes a barrier and protects further attack by the acid. The inhibitor was found to have good efficiency at all immersion periods.
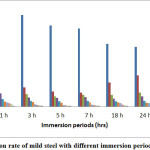 |
Figure 1: corrosion rate of mild steel with different immersion periods Click here to View Figure |
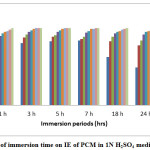 |
Figure 2: Effect of immersion time on IE of PCM in 1N H2SO4 medium Click here to View Figure |
Table 1: CR of mild steel and IE of PCM on mild steel in 1N H2SO4 at different immersion periods
|
S.No |
Conc. of PCM %v/v |
1h |
3h |
5h |
7h |
18h |
24h |
||||||
|
CR (mpy) |
IE (%) |
CR (mpy) |
IE (%) |
CR (mpy) |
IE (%) |
CR (mpy) |
IE (%) |
CR (mpy) |
IE (%) |
CR (mpy) |
IE (%) |
||
|
1 |
Blank |
1290 |
– |
1850 |
– |
1640 |
– |
1579 |
– |
1271 |
– |
1210 |
– |
|
2 |
0.01 |
470 |
63 |
388 |
79 |
308 |
81 |
280 |
82 |
489 |
61 |
635 |
48 |
|
3 |
0.05 |
313 |
75 |
252 |
86 |
210 |
87 |
240 |
84 |
240 |
81 |
280 |
76 |
|
4 |
0.1 |
266 |
79 |
183 |
90 |
177 |
89 |
196 |
87 |
162 |
87 |
210 |
82 |
|
5 |
0.5 |
152 |
88 |
116 |
93 |
110 |
93 |
118 |
92 |
112 |
91 |
114 |
90 |
|
6 |
1 |
104 |
91 |
91 |
95 |
81 |
95 |
86 |
94 |
83 |
93 |
80 |
93 |
|
7 |
1.5 |
87 |
93 |
47 |
97 |
37 |
97 |
38 |
97 |
71 |
94 |
70 |
94 |
|
8 |
2 |
70 |
94 |
33 |
98 |
25 |
98 |
32 |
97 |
41 |
96 |
51 |
95 |
|
9 |
2.5 |
58 |
96 |
27 |
98 |
18 |
98 |
23 |
98 |
28 |
97 |
30 |
97 |
|
10 |
3 |
21 |
98 |
21 |
98 |
16 |
98 |
19 |
98 |
21 |
98 |
22 |
98 |
Polarization results
The effect of PCM concentration on the corrosion behavior of mild steel in 1N sulfuric acid solution was studied by polarization measurements. From polarization curves, the electrochemical parameters as obtained are shown in table 2. These include corrosion potential (Ecor), corrosion current density (Icor) determined by the cathodic Tafel line to the corrosion potential. The cathodic (βc) Tafel slope decreases suggesting that the extract inhibits the corrosion of mild steel through the adsorption of its secondary metabolites and it acts as a mixed type inhibitor. The values of Ecorr change slowly to negative values and the value of Icorr decreases and hence the value of inhibition efficiency increases indicating the inhibiting effect of PCM.
Table 2: calculated polarization parameters for mild steel in 1N H2SO4 for various concentrations of PCM
| Concentration of PCM %v/v | βa | βc | Rp | Icorr mA cm-2 | Ecorr mV |
IE % |
|
| From Icorr | From Rp | ||||||
|
Blank |
246 |
323 |
20.29 |
2.989 |
-458 |
– |
– |
|
0.05 |
188 |
244 |
64.33 |
0.717 |
-462 |
76 |
68 |
|
0.1 |
192 |
257 |
76.03 |
0.628 |
-464 |
78 |
73 |
|
0.5 |
178 |
263 |
128.60 |
0.359 |
-452 |
87 |
84 |
|
1.0 |
158 |
255 |
176.50 |
0.240 |
-464 |
91 |
88 |
|
1.5 |
166 |
249 |
206.67 |
0.210 |
-481 |
92 |
90 |
|
2.0 |
174 |
239 |
244.26 |
0.179 |
-475 |
94 |
91 |
|
2.5 |
182 |
223 |
488.98 |
0.0897 |
-462 |
96 |
95 |
|
3.0 |
185 |
228 |
727.24 |
0.0612 |
-469 |
97 |
97 |
Electrochemical impedance measurement
The Nyquist plot of mild steel in 1N H2SO4 and in the presence of different concentrations of PCM are given in Figure 3. The impedance parameters are summarized in Table 3. The impedance diagram showed depressed semicircles indicating the corrosion of mild steel was controlled by charge transfer process. The diameter of the capacitive loop in the presence of PCM is larger than that in the blank solution. The values of charge transfer resistance (Rct) that increased with inhibitor concentrations revealed that the formation of a protective film on the mild steel surface. The values of surface in homogeneity coefficient (n) decreased with increasing concentration of PCM extract. The Cdl values shown in the Table 3 are found to decrease with increasing extract concentration. This confirms that the plant constituents are adsorbed on the metal surface resulting in decrease in double layer capacitance. The increasing charge transfer resistance Rct values showed the reduced corrosion rate in the presence of the plant extract. It was confirmed that the plant extract shows good corrosion inhibition efficiency.
Table 3: Impedance parameters for corrosion of mild steel in 1N H2SO4 without and with different concentrations of PCM.
| Concentration of PCM %v/v | Rct ohmcm2 |
n |
Cdl µFcm2 | IE%From Rct |
|
Blank |
17.3 |
0.935 |
122.8 |
– |
|
0.05 |
72.1 |
0.932 |
92.5 |
76 |
|
0.1 |
82.4 |
0.902 |
85.2 |
79 |
|
0.5 |
144.2 |
0.865 |
71.3 |
88 |
|
1.0 |
216.3 |
0.844 |
57.4 |
92 |
|
1.5 |
247.1 |
0.841 |
40.2 |
93 |
|
2.0 |
288.3 |
0.836 |
29.4 |
94 |
|
2.5 |
577.2 |
0.825 |
18.2 |
97 |
|
3.0 |
910.5 |
0.821 |
10.3 |
98 |
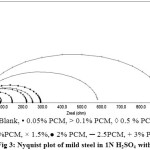 |
Figure 3: Nyquist plot of mild steel in 1N H2SO4 without and with different concentrations of PCM. |
SEM Analysis
SEM micrographs of mild steel surfaces after immersion for 3h in the presence and absence of PCM extract are shown in Fig 4a, 4b and 4c. Fig 4a shows the SEM image of polished mild steel surface. Fig 4b showed that mild steel coupon in uninhibited 1N H2SO4 was highly damaged; it can be concluded that MS surface was highly corroded in the absence of inhibitor. Fig 4c shows that there is less damage on the MS surface in the presence of 3% of PCM extract; which further confirms the adsorption behavior of inhibitor on MS surface.
 |
Figure 4a: Polished Mild Steel Click here to View figure |
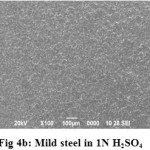 |
Figure 4b: Mild steel in 1N H2SO4 Click here to View Figure |
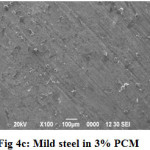 |
Figure 4c: Mild steel in 3% PCM Click here to View Figure |
Conclusions
The finding of the study shows that
1. PCM acts as green inhibitor for the corrosion of mild steel in 1N H2SO4.
2. It was found that the corrosion rate of mild steel decreases with the increase in concentration of the inhibitor.
3. The results of weight loss measurements for different immersion periods at room temperature show the maximum inhibition efficiency of 98% at 3% v/v of PCM.
4. The effect of immersion time on inhibition efficiency shows that the inhibitor is effective even for longer immersion periods at low concentration.
5. Polarization results showed that the PCM is a mixed type inhibitor.
6. According to impedance study the increasing charge transfer resistance Rct values showed the reduced corrosion rate in the presence of the plant extract.
7. SEM analysis revealed that the adsorption of secondary metabolites in the plant extract on the mild steel surface which is responsible for the inhibition of corrosion.
Acknowledgements
The author is thankful to her respective management and gratefully acknowledges the financial support of the University Grants Commission (UGC) of Hyderabad (Project No. 4-4/2014-15(MRP-SEM/UGC-SERO).
References
- Elyn Amira ,W.A.W.; Rahim ,A.A.; Osman ,H.; Awang, K.; Bothi Raja, P. Int. J. Electrochem. Sci. 2011, 6, 2998 – 3016.
- Rajendran, A.; Karthikeyan, C. International Journal of Plant Research. 2012, 2(1), 9-14
- Manoj Acharya; Jinendra Singh Chouhan; Anita Dixit; Gupta, D. K. Chemistry and Materials Research. 2013, 3, No.6.
- Saratha ,R.; Vasudha, V.G. E-Journal of Chemistry. 2009, 6(4), 1003-1008.
CrossRef - Abu-Dalo, M.A.; Othman, A.A.; Al-Rawashdeh, NAF. Int. J. Electrochem. Sci. 2012, 7, 9303-9324.
- Cang Hui; Fei Zhenghao; Shao Jinling; Shi Wenyan; Xu Qi. Int. J. Electrochem. Sci. 2013, 8, 720-734.
- Vasudha, V.G.; Saratha, R. Orient. J. Chem. 2011, 27(3), 1165-1171.
- Khandelwal, R.; Arora, S. K.; Mathur, S.P. Asian Journal of Biochemical and Pharmaceutical Research Issue. 2014, 1(4), 115-128.
- Raja, P. B.; Sethuraman, M.G. J.Mater. Eng. Perform. 2010, 19, 761-766.
CrossRef - Deng, S.; Li, X. Corros. Sci. 2012, 55, 407-415.
CrossRef - Saedah R. Al-Mhyawi. Orient. J. Chem. 2014, 30(2), 541-552.
CrossRef - Ghadah M. Al-Senani; Sameerah I. Al-Saeedi ; Rasmiah Almufarij.Orient. J. Chem. 2015,31(4), 2077-2086.
CrossRef - Patel, N. S.; Jauhari, S.; Mehta, G. N. The Arabian Journal for Science and Engineering. 2009, 34, Number 2C
- Park, Y.K.; Cha, H.S.; Park, M.W.; Kang, Y.H.; Seog, H.M. J Korean Soc Food Sci Nutr. 1997, 26, 639–646.
- Anbarasi, .K.; Vasudha, V.G. Chem Sci Rev Lett .2014,3(9), 45-51.
- Saedah R. Al-Mhyawi. Afr. J. Pure Appl.Chem. 2014, 8 (1), 9-22.
CrossRef - Saratha, R.;Vasudha, V.G. E.journal of Chemistry ,2010,7(3),677-684.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









