Electrocatalytic Activity and Electrochemical Impedance Spectroscopy of Poly(Aniline-Co-Ortho-Phenylenediamine) Modified Electrode on Ascorbic Acid
Ali Parsa* and Sara Amanzadeh-Salout
Department of Chemistry, College of Science, Yadegar -e- Imam Khomeini (RAH) Shahre-rey Branch, Islamic Azad University, Tehran, Iran.
Corresponding Author E-mail: aliparsa@iausr.ac.ir
DOI : http://dx.doi.org/10.13005/ojc/320432
Article Received on :
Article Accepted on :
Article Published : 20 Jul 2016
The poly(aniline-co-ortho-phenylenediamine) modified composite graphite (poly(Ani–co–oPDA)/CG)) has shown excellent electrocatalytic response towards the oxidation of ascorbic acid (AA). The anodic peak potential (Epa) of AA has shifted from +0.48 V (bare CG) to +0.17 V (poly(Ani–co–oPDA/CG)). The anodic peak currents (Ipa) are linearly dependent upon the square root of scan rate indicating a favourable diffusion controlled process. The electro oxidation of AA on poly(Ani–co–oPDA/CG is more feasible in acidic medium than in either neutral or alkaline medium. This is shown by negative shift of Epa. The charge transfer resistance (Rct) at the poly(Ani–co–oPDA/CG) shows that the rate of the electro oxidation of AA changes with electrode potential. The Rct and diffusion process are dependant not only on applied potential and electrode material but also on the AA. The poly(aniline-co-ortho-phenylenediamine) modified composite graphite (CG) electrode has shown excellent electrocatalytic response towards the oxidation of ascorbic acid (AA). Charge transfer resistance (Rct) at the poly(Ani–co–oPDA/CG) shows that the rate of the electro oxidation of AA changes with electrode potential.
KEYWORDS:Ascorbic acid; Electrochemical impedance spectroscopy; Electrocatalytic oxidation; Ortho–Phenylenediamine
Download this article as:| Copy the following to cite this article: Parsa A, Amanzadeh-Salout S. Electrocatalytic Activity and Electrochemical Impedance Spectroscopy of Poly(Aniline-Co-Ortho-Phenylenediamine) Modified Electrode on Ascorbic Acid. Orient J Chem 2016;32(4). |
| Copy the following to cite this URL: Parsa A, Amanzadeh-Salout S. Electrocatalytic Activity and Electrochemical Impedance Spectroscopy of Poly(Aniline-Co-Ortho-Phenylenediamine) Modified Electrode on Ascorbic Acid. Orient J Chem 2016;32(4). Available from: http://www.orientjchem.org/?p=19092 |
Introduction
The determination of ascorbic acid (AA) has become very important since AA is known to be present in human brain and also involves in several biological processes 1.Recent studies have demonstrated that the AA content in biological fluids is useful in assessing the amount of oxidative stress in human metabolism 2.The excessive oxidative stress has been linked to cancer, diabetes mellitus and hepatic disease.
Voltametric studies of AA on bare electrode have not been very successful 3-6. The main cause is the high over potential of AA besides the fouling effect of the oxidation products, poor reproducibility, low selectivity and poor sensitivity. However, this is overcome by modifying the electrode with mediators 7-9 and polymers 10-12.
The electrochemical copolymerization of aniline (Ani) with its derivatives has been reported 13-19 These have significantly improved the electrochemical activity of polyaniline (PAni). The electro copolymerization of Ani with other monomers is, however, not feasible and even in some cases impossible. The reason is large peak potentials separation (∆Ep) between them which hindered the formation of the copolymer. Hence, the smaller the ∆Ep the better it is for the copolymerization to occur 20-24.
The electrochemical impedance spectroscopy (EIS) is a useful technique to investigate the interfacial properties of modified electrodes 25-30. The EIS is, usually, utilized to study parameters such as charge transfer resistance (Rct), double layer capacitance (Cdl), faradaic capacitance etc. of modified electrodes 31-34. EIS on PAni and its derivatives modified electrodes have been reported 34-37
This work reports on the electrochemical behaviour and catalytic ability of poly(Ani–co–oPDA) modified CG electrode towards electro-oxidation of AA.
 |
Graphical Abstract |
Materials and Methods
Materials
Aniline (Sigma Chemicals, USA) was purified by distillation under a nitrogen atmosphere at reduced pressure. The resulting colorless liquid was kept in the dark at 5 °C. The ortho–phenylenediamine (oPDA) and phosphoric acid (Sigma Chemicals, USA) were used as received. Ascorbic acid (AA) (Fluka Chemical, Switzerland) solution was prepared fresh in 0.1 mol L−1 phosphate buffer solution, pH 6.8. All aqueous solutions were prepared using ultra pure water from Milli Q plus system (Millipore Corp., USA). Oxygen-free nitrogen (OFN) was obtained from Nissan-IOI, Malaysia.
Equipment
CV was carried out using an electrochemical Autolab PGSTAT system (Eco. Chemie B.V., Netherlands). The system was run on a PC using general-purpose electrochemical system (GPES 4.9) software. A three-compartment electrochemical cell was employed during synthesis and characterization of copolymer. The 2B pencil composite graphite (CG) Lumograph (Staedtler, Germany) was used as working and counter electrodes against pseudo Ag/AgCl reference electrode.EIS measurements were taken using the CompactStatpotentiostat (CompactStat; Ivium Technologies, Netherlands) controlled by a computer equipped with the IviumSoft software package. The structure of copolymer was determined by FTIR spectroscopy System 2000 (Perkin Elmer, USA).
Procedure
The electro copolymerization was performed using 25 mL solution of the 50 m mol L-1 Ani and oPDA, 1 mol L-1 H3PO4 and 0.06 mol L-1 Ca3(PO4)2 by sweeping the potential between −0.7 V and +0.8 V (vs. Ag/AgCl), at a scan rate 100 mV s−1, under OFN atmosphere and at 25 ± 2 ºC. The electrochemical determination of AA was performed using 25 mL solution containing 2 m mol L-1 AA and 1 mol L-1 H3PO4 by sweeping the potential between −0.45 V to +0.65 V at a scan rate 100 mVs−1, under OFN atmosphere and at 25 ± 2 ºC.
Copolymer film was repeatedly washed with supporting electrolyte and then placed in monomer free supporting electrolyte containing AA at different applied dc potentials to perform ac impedance measurements. The potential amplitude of ac impedance was kept at 10 mV with its frequency range from 100 kHz to 10 mHz.
Results and Discussion
Electrosynthesis of copolymer Ani and oPDA
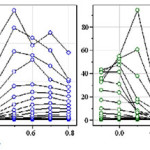
The CVs of copolymerization of Ani and oPDA in 1 mol L-1 H3PO4 containing 0.06 mol L-1 Ca3(PO4)2 is shown in Figure 1a. The details have been reported elsewhere 38. Figure 1b shows FT-IR spectra bands at 1634–1618 cm−1 indicating the existence of oPDA in the copolymer 39. The appearance of bands at 1068–1064, 884 and 850 cm−1 suggests the presence of phenazine-like cyclic structures in the copolymer backbone 40. These could either be due to the presence of PoPDA blocks or of the adjacent oPDA and Ani unit in the copolymeric chain 38.
 |
Figure 1: (a) The CVs of 50 mmol L-1Ani and 50 mmol L-1 oPDA in 1 mol L-1 H3PO4 containing 0.06 mol L-1 Ca3(PO4)2.The applied potential, Eapp, −0.7 and +0.8 V (vs. Ag/AgCl),scan rate of 100 mVs−1 and scanning up to 10 cycles.(b) Baseline corrected FTIR spectrum of copolymer. |
Electrocatalytic oxidation of AA
Figure 2c shows that the anodic peak potential (Epa) of AA appears at +0.48 V on bare CG with the anodic peak current (Ipa) at 5 mA. It is an irreversible electrode process. In contrast, on poly(Ani–co–oPDA)/CG the Ipa has increased to 9 mA and the Epa has shifted negatively to +0.17 V (Fig. 2d) with quasi reversible electrode process. This shows that the electro oxidation of AA is feasible indicating a strong electrocatalytic property of poly(Ani–co–oPDA)/CG towards AA. The shifting of the Epa is due to the kinetic effect in which a substantial increase in the rate of electron transfer is observed 41.
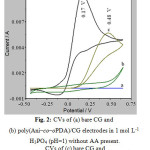 |
Figure 2: CVs of (a) bare CG and(b) poly(Ani–co–oPDA)/CG electrodes in 1 mol L-1H3PO4 (pH=1) without AA present.CVs of (c) bare CG and(d) poly(Ani–co–oPDA)/CG electrodes in 1 mol L-1 H3PO4(pH 1) in the presence of 2 mmol L-1 AA |
Rueda et al 42 have studied the oxidation of AA on a gold electrode in a wide pH range. The products of the reaction have been identified by chromatography. From electrochemical experiments, the total number of electrons taking part in the oxidation has been estimated at 1.9. On this basis the following EC mechanism have been proposed:
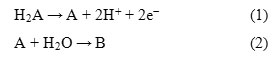
Where H2A is AA, A stands for the dehydro ascorbic acid (DHAA) and B is hydrated dehydro ascorbic acid (DHAA.H2O). The DHAA is formed from H2Avia a radical anion intermediate viz. mono dehydro ascorbic acid. The DHAA undergoes a hydration reaction to form the final product, the electro inactive DHAA.H2O. The irreversibility of the electro oxidation of AA is displayed by the absence of the cathodic peak current (Ipc) (Figure 2).
Effect of scan rate
Further investigation is carried out on the transport characteristics of AA on the modified electrodes. It is apparent that with increasing scan rates the Epa is shifted to more positive potentials indicating there is kinetic limitation on the electrode process (Figure 3). But, as Ipa is directly proportional to the square root of scan rates the electrode process is, predominantly, diffusion-controlled 43. This is regarded as normal for a useful electrode process.
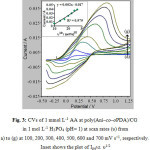 |
Figure 3: CVs of 1 mmol L-1 AA at poly(Ani–co–oPDA)/CGin 1 mol L-1 H3PO4 (pH= 1) at scan rates (υ) from a) to (g) at 100, 200, 300, 400, 500, 600 and 700 mV s−1, respectively.Inset shows the plot of Ipavs. υ1/2 |
Effect of pH
The CVs of 2 m mol L-1 AA in supporting electrolytes at different pH values at poly(Ani–co–oPDA)/CG are shown in Figure 4. It appears that there is a decrease in Ipa when pH is raised from 1 to 12. The decrease in the electrode process is due to higher electronic conductivity of poly(Ani–co–oPDA) in acid than in basic media 44, 45.With an increase in pH the Epa is shifted towards positive indicating the decrease in electro catalysis of poly(Ani–co–oPDA)/CG. Hence, the electro oxidation of AA on the poly(Ani–co–oPDA)/CG is more feasible in acid than in either neutral or alkaline media. Therefore, the poly(Ani–co–oPDA)/CG is useful for electro oxidation of AA in acidic pH range.
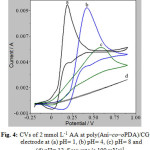 |
Figure 4: CVs of 2 mmol L-1 AA at poly(Ani–co–oPDA)/CG electrode at (a) pH= 1, (b) pH= 4, (c) pH= 8 and (d) pH= 12. Scan rate is 100 mVs−1. |
EIS studies
It is well known that EIS is a useful method for studying the interfacial properties of the modified electrodes 46. The impedance is the summation of real, Zre, and imaginary, Zim, components contributed by the resistance and capacitance of the cell 47.In this study EIS was employed to investigate electro oxidation of AA at poly(Ani–co–oPDA)/CG and bare CG electrodes. EIS measurements of AA in a monomer-free supporting electrolyte solution were performed at two different potentials.
From Figure 5a it is obvious that the magnitude of charge transfer resistance (Rct), the diameter of the semi circle, of poly(Ani–co–oPDA)/CG is smaller (2.900×103 ohm) than bare CG (2.750×104 ohm) indicating improved interfacial capacitance. In short, double-layer capacitance (Cdl) of constant phase element (CPE or Q) has encouraged charge transfer. The presence of straight line on poly(Ani–co–oPDA)/CG with a slope of 45o at the lower frequency indicates a favourable diffusion-controlled mass transport process 48.
 |
Figure 5: (a) Nyquist plots of AA at the poly(Ani–co–oPDA)/CG and bare CG electrodes at Eapp0.20 V. (b) and (c) are the respective electrodes with Randle’s equivalent circuit and circuit description code (CDC) (inset). The ac potential amplitude |
description code (CDC) (inset). The ac potential amplitude was kept at 10 mV and frequency range used was from 100 kHz to 10 mHz.
The total impedance is measured by several parameters: the bulk electrolyte solution resistance (Rs), charge transfer resistance (Rct) that corresponds to the kinetic control of the charge-transfer process, double layer capacitance (Cdl) and Warburg impedance (Zw). To take a proper fitting of the Nyquist plots, it was needed to replace Cdl with a CPE in the Randles’ equivalent circuit. It’s widely believed explanation for the appearance of depressed semi circles and the presence of CPE in the Nyquist plots is due to microscopic roughness, which causes an inhomogeneous distribution in both Cdl and Rs 49-51. The circuit element that is of most interest to this work is Rct. This is because it often relates directly to the accessibility of the modified electrode and also reflects the flow of charge across the modified interface into the substrate electrode.
The decrease in diameter of the semicircle confirms the electrocatalytic activity of poly(Ani–co–oPDA)/CG in the oxidation of AA at Eapp 0.2 V. The electro oxidation of AA that occurred via phenazine-like cyclic species virtually led to an increase in the surface concentration of low-valence species of the electrocatalyst and the decline of the Rct depending on the concentration of AA in the solution as shown in Figure 5c, Rct is high enough at 0.2 V, where the mass transfer process is not performed, so in the Nyquist plots no Zw is observed.
At Eapp 0.5 V the result shows the inverse as magnitude of Rct of poly(Ani–co–oPDA)/CG (15000 ohm) is much higher than the bare CG (3400 ohm) and with Zw is not observed (Figure 6b). This suggests on the dependancy of diffusion process of AA on the Eapp. Values of the Randles equivalent circuit elements obtained by fitting the experimental results for the Nyquist plots in monomer free background electrolyte containing AA at the poly(Ani–co–oPDA)/CG and bare CG at Eapp of 0.2 and 0.50 V are listed in Table 1.
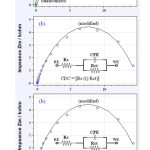 |
Figure 6: (a) Nyquist plots of AA at the poly(Ani–co–oPDA)/CG and bare CG electrodesat Eapp0.50 V. (b) and (c) are the respective electrodes with Randle’s equivalent circuit and circuit description code (CDC) (inset). The ac potential amplitude was kept at 10 mV and frequency range used was from 100 kHz to 10 mHz. |
Table 1: The best fitting values of the Randles’ equivalent circuit elements in Figures 5 and 6 from the simulation of the impedance data for poly(Ani–co–oPDA)/CG and CG electrodes.
| Eapp (V) | 0.2 | 0.5 | |||||||||||||
| Electrode | Modified | Unmodified | Modified | Unmodified | |||||||||||
| Rs (ohm)Rct (ohm)W 1/(Ohm sqrt(Hz)CPE (F)α | 102.900×1035.950×1021.650×10-67.200×10-1 | 2.620×1012.750×104————-1.810×10-58.105×10-1 | 7.201.500×104————–4.500×10-76.805×10-1 | 4.000×1013.400×1031.200×1023.000×10-57.950×10-1 | |||||||||||
α: Degree of electrode surface roughness or Cell geometry.
Further investigation shows that the poly(Ani–co–oPDA)/CG electrode has minimum Zre at Eapp 0.2 V, whereas the CG electrode shows minimum Zre at Eapp 0.5 V at frequency 100 kHz to 10 mHz (Figures 7a and 7b). Figure 7c shows real impedance spectroscopy (Zre or Z׳) of poly(Ani–co–oPDA)/CG electrode in monomer free background electrolyte containing uric acid (UA) obtained by sweeping the potential between −0.1 and 0.8 V (vs. Ag/AgCl). The poly(Ani–co–oPDA)/CG electrode has its minimum Zre at Eapp 0.3 V. Thus, the Rct and diffusion process depend not only on Eapp and electrode material but also on type of analyte available.
 |
Figure 7: Real impedance spectroscopy (Zre) of poly(Ani–co–oPDA)/CG in monomer free background electrolyte containing(a) AA and (c) UA obtained by sweeping the potential between −0.1 and 0.8 V (vs. Ag/AgCl). Real impedance spectroscopy (Zre) of bare CG electrode in the same environment as the above. |
Conclusion
The poly(Ani–co–oPDA)/CG electrode is shown to possess catalytic activity towards the oxidation reaction of AA. As a result, the electro oxidation of AA on the modified electrode is more feasible in acidic than in neutral and alkaline medium. This is shown by negative shift of Epa of AA. The extension of catalytic reaction depends on charge transfer. The electrode process is diffusion-controlled. The study indicates the poly(Ani–co–oPDA)/CG electrode is useful for oxidation of AA in an acidic range of pH.
The electrochemical impedance spectroscopy was successfully used to interpret the electrocatalytic effect of modified electrode on AA. The equivalent circuit elements obtained by fitting the experimental results confirmed that proposed equivalent circuits were matched and able to explain phenomenon in the modified and unmodified electrode. The Rct and diffusion process are not only dependent on applied potential and electrode material but also on type of analyte used. The Rct is the only circuit element that is meaningful. It describes on the kinetic of charge transfer during the electro oxidation of AA at different applied potential.
Acknowledgment
This work is supported by Yadegar -e- Imam Khomeini (RAH) Shahre-rey Branch, Islamic Azad University, Tehran, Iran for Research University (RU) grant – 1395/1742:90/07/27.
References
- Männistö, P. T.; Kaakkola, S. Pharmacol. Rev. 1999, 51, 593-628 .
- Koshiishi, I.; Imanari, T. Anal. Chem. 1997, 69, 216-220 .
CrossRef - Kalakodimi, R. P.; Nookala, M. Anal. Chem. 2002, 74, 5531-5537 .
CrossRef - Khoo, S. B.; Chen, F. Anal. Chem. 2002, 74, 5734-5741 .
CrossRef - Lertanantawong, B.; O’Mullane, A. P.; Zhang, J.; Surareungchai, W.; Somasundrum, M.; Bond, A. M. Anal. Chem. 2008, 80, 6515-6525 .
CrossRef - Malinauskas, A.; Garjonyt, R.; Mažeikien, R.; Jurevičiūt, I. Talanta. 2004, 64, 121-129 .
CrossRef - Balamurugan, J.; Senthil Kumar, S. M.; Thangamuthu, R.; Pandurangan, A. J. Mol. Catal. A: Chem. 2013, 372, 13-22 .
CrossRef - Wei, M.-Y.; Huang, R.; Guo, L.-H. J. Electroanal. Chem. 2012, 664, 156-160 .
CrossRef - Wu, M.; Mao, X.; Li, X.; Yang, X.; Zhu, L. J. Electroanal. Chem. 2012, 682, 1-6 .
CrossRef - Abdelwahab, A. A.; Kim, D.-M.; Halappa, N. M.; Shim, Y.-B. Electroanalysis. 2013, 25, 1178-1184 .
CrossRef - Chih, Y.-K.; Yang, M.-C. Bioelectrochemistry. 2013, 91, 44-51 .
CrossRef - Kalimuthu, P.; John, S. A. Bioelectrochemistry. 2009, 77, 13-18 .
CrossRef - Mu, S. Synth. Met. 2004, 143, 259-268 .
CrossRef - Sato, M.; Yamanaka, S.; Nakaya, J.-i.; Hyodo, K. Electrochim. Acta. 1994, 39, 2159-2167 .
CrossRef - Shah, A.-u.-H. A.; Holze, R. J. Solid State Electrochem. 2007, 11, 38-51 .
CrossRef - Si, S.; Xu, Y.; Nie, L.; Yao, S. Electrochim. Acta. 1995, 40, 2715-2721 .
CrossRef - Tang, H.; Kitani, A.; Maitani, S.; Munemura, H.; Shiotani, M. Electrochim. Acta. 1995, 40, 849-857 .
CrossRef - Wei, Y.; Hariharan, R.; Patel, S. A. Macromolecules. 1990, 23, 758-764 .
CrossRef - Yang, C. H.; Wen, T. C. J. Appl. Electrochem. 1994, 24, 166-178 .
CrossRef - Su, N. Nanoscale Res. Lett. 2015, 10, 1-9 .
CrossRef - Ojani, R.; Hamidi, P.; Raoof, J.-B. Chin. Chem. Lett. 2016, 27, 481-486 .
CrossRef - Rezaei, B.; Boroujeni, M. K.; Ensafi, A. A. Sensors Actuators B: Chem. 2016, 222, 849-856 .
CrossRef - Morteza, E.; Roya, D. Oriental Journal of Chemistry. 2015, 31, 1185-1189 .
- Udmale, V.; Mishr, D.; Gadhave, R.; Pinjare, D.; Yamgar, R. Oriental Journal of Chemistry. 2013, 29, 927-936 .
CrossRef - Arslan, A.; Hur, E. Chemical Papers. 2014, 68, 504-515 .
- Atta, N. F.; El-Kady, M. F.; Galal, A. Anal. Biochem. 2010, 400, 78-88 .
CrossRef - Atta, N. F.; Galal, A.; Khalifa, F. Appl. Surf. Sci. 2007, 253, 4273-4282 .
CrossRef - Branzoi, V.; Branzoi, F.; Pilan, L. Mater. Chem. Phys. 2009, 118, 197-202 .
CrossRef - Manjunatha, R.; Suresh, G. S.; Melo, J. S.; D’Souza, S. F.; Venkatesha, T. V. Sensors Actuators B: Chem. 2010, 145, 643-650 .
CrossRef - Wang, X.; Bernard, M. C.; Deslouis, C.; Joiret, S.; Rousseau, P. Electrochim. Acta. 2011, 56, 3485-3493 .
CrossRef - Kim, Y.-R.; Bong, S.; Kang, Y.-J.; Yang, Y.; Mahajan, R. K.; Kim, J. S.; Kim, H. Biosens. Bioelectron. 2010, 25, 2366-2369 .
CrossRef - Liu, X.-X.; Dou, Y.-Q.; Wu, J.; Peng, X.-Y. Electrochim. Acta. 2008, 53, 4693-4698 .
CrossRef - Gobal, F.; Faraji, M. Electrochim. Acta. 2013, 100, 133-139 .
CrossRef - Córdoba-Torres, P.; Mesquita, T. J.; Devos, O.; Tribollet, B.; Roche, V.; Nogueira, R. P. Electrochim. Acta. 2012, 72, 172-178 .
CrossRef - Hallik, A.; Alumaa, A.; Tamm, J.; Sammelselg, V.; Väärtnõu, M.; Jänes, A.; Lust, E. Synth. Met. 2006, 156, 488-494 .
CrossRef - Lissy, S. L. G.; Pitchumani, S.; Jayakumar, K. Mater. Chem. Phys. 2002, 76, 143-150 .
CrossRef - Ameen, S.; Shaheerakhtar, M. Oriental Journal of Chemistry. 2013, 29, 837-860 .
- Parsa, A.; Ab Ghani, S. Polymer. 2008, 49, 3702-3708 .
CrossRef - Huang, M. R.; Li, X. G.; Duan, W. Polym. Int. 2005, 54, 70-82 .
CrossRef - Murugesan, R.; Subramanian, E. Mater. Chem. Phys. 2003, 80, 731-739 .
CrossRef - Zhang, L.; Lian, J. J. Electroanal. Chem. 2007, 611, 51-59 .
CrossRef - Rueda, M.; Aldaz, A.; Sanchez-Burgos, F. Electrochim. Acta. 1978, 23, 419-424 .
CrossRef - Hu, Z.-A.; Shang, X.-L.; Yang, Y.-Y.; Kong, C.; Wu, H.-Y. Electrochim. Acta. 2006, 51, 3351-3355 .
CrossRef - Nekrasov, A. A.; Ivanov, V. F.; Vannikov, A. V. Electrochim. Acta. 2001, 46, 4051-4056 .
CrossRef - Ostwal, M. M.; Qi, B.; Pellegrino, J.; Fadeev, A. G.; Norris, I. D.; Tsotsis, T. T.; Sahimi, M.; Mattes, B. R. Ind. Eng. Chem. Res. 2006, 45, 6021-6031 .
CrossRef - Feng, L.-J.; Zhang, X.-H.; Zhao, D.-M.; Wang, S.-F. Sensors Actuators B: Chem. 2011, 152, 88-93 .
CrossRef - Qiu, S.; Gao, S.; Liu, Q.; Lin, Z.; Qiu, B.; Chen, G. Biosens. Bioelectron. 2011, 26, 4326-4330 .
CrossRef - Kear, G.; Barker, B.; Stokes, K.; Walsh, F. Electrochim. Acta. 2007, 52, 2343-2351 .
CrossRef - Cattarin, S.; Musiani, M. Electrochim. Acta. 2007, 52, 2796-2805
CrossRef - Jung, M.-H.; Lee, H. Langmuir. 2008, 24, 9825-9831 .
CrossRef - Lee, C. Mater. Chem. Phys. 2009, 114, 125-133 .
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









