A Study on Nitrate Ion Reduction in Water with Zero-Valent Iron Loaded Nano-Titania Prepared from Ilmenite Ore
M.H.H.Mahmoud1,2,*
1Materials Science and Engineering Group, Chemistry Department, College of Science, Taif University, Taif, Kingdom of Saudi Arabia.
2Central Metallurgical Research and Development Institute, P.O.Box, 87, Helwan, Cairo, Egypt.
*Corresponding Author Email: mheshamm@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/320424
This paper aims to synthesize titanium dioxide loaded with nano-zero valent iron (nZVI-TiO2) from an inexpensive ilmenite ore. Ilmenite leachant, a solution rich in both titanium and iron ions,has been prepared by leaching ilmenite ore in hydrochloric acid. At 30% HCl, 70 °C and 5 hours, almost all of iron and titanium in the ilmenite ore were dissolved. Nano titania was then produced by hydrolysis of the Ilmenite leachant in boiling water under open atmosphere and nano-zero valent iron (nZVI) was subsequently precipitated on its surface by reduction with sodium borohydride. The synthesized nZVI-TO2was characterized by TEM, UV-Vis absorption, XRD and EDXRF and tested for reduction of nitrate ion from water. The synthesized material showed good performance in reduction of nitrate ion in water compared with nZVI.About 40% of nitrate ion could be removed using nZVI-TO2from nearly neutral water after 3 hours.
KEYWORDS:Nitrate ion; Reduction; Nano- Zero valent Iron; Ilmenite ore
Download this article as:| Copy the following to cite this article: Hesham M, Mahmoud H. A Study on Nitrate Ion Reduction in Water with Zero-Valent Iron Loaded Nano-Titania Prepared from Ilmenite Ore. Orient J Chem 2016;32(4). |
| Copy the following to cite this URL: Hesham M, Mahmoud H. A Study on Nitrate Ion Reduction in Water with Zero-Valent Iron Loaded Nano-Titania Prepared from Ilmenite Ore. Orient J Chem 2016;32(4). Available from: http://www.orientjchem.org/?p=20847 |
Introduction
Nitrate ion are considered as hazardous environmental contaminants of ground and surface water. According to the US Environmental Protection Agency (EPA), nitrate ion threshold concentration in drinking water should not exceed 10 mg/ Lit 1. However, this threshold level is surpassed in many cases due to many reasons, the most important being due to the intensive and uncontrolled use of fertilizers in agriculture [2]. Unfortunately, nitrate ions may persist for decades in groundwater and accumulate to high levels as more nitrogen based fertilizers are fed to the soil each year[3]. Various techniques have been adopted so far for drinking water denitrification: ion-exchange, reverse osmosis, adsorption, biological denitrification and chemical reduction[4-6]. These techniques have their advantages and disadvantages. Ion exchange and reverse osmosis suffer from a medium to high operating cost due to the frequent regeneration of the media and production of secondary brine waste. Adsorption by ion exchangers suffers from strong pH and temperature dependency and spent adsorbent disposal problem [7]. Biological denitrification has a lower operating cost and is the prevalent method used for the moment. It suffers from excessive biomass and soluble microbial by-products. In addition, this process is relatively slow and, compared to the chemical reduction method, sometimes suffer from incomplete removal of nitrate ion[8].
Nano sized zero valent metals possess a high specific surface area and a high surface reactivity. A technical problem associated with the industrial application of such materials is that they cannot be used alone and should be supported. Pillared clays and zeolites have been recently used as low cost effective supports for nano-scale zero valent iron [9].Nano-sized support materials for zero valent iron can help in increasing the surface area without agglomeration. Nano titania is suggested to be a suitable support due to its high chemical stability at different aqueous media. In this work, ilmenite ore has been used to prepare nano-titania.
Natural ilmenite is an inexpensive raw material contains varieties of components such as TiO2,FeO, Fe2O3 and SiO2. The extraction methods of titanium from ilmenite can be summarized into two categories; pyrometallurgical and hydrometallurgical routes. Hydrochloric acid leaching is much simpler and the residual acid can be recycled to the process10-13. The titanium acidic solution shouldbefurther purified and hydrolyzed to produce pure TiO214.
We have previously utilized an Egyptian ilmenite ore for preparation of nano-titania by acid leaching, purification for separation of iron and hydrolysis. The prepared material was used as a catalyst loaded with different metal ions for removal of varieties of pollutants in water 15,16.
Since zero-valent iron (Fe(0)) was first acknowledged as a treatment medium for environmental remediation nearly two decades ago 17, it has been advanced into a viable and attractive option for treatment of many types of organic and inorganic contaminants 18. Removal of contaminants by Fe(0) occurs via several physicochemical processes including chemical reduction, specific and nonspecific adsorption and coprecipitation. Ryu et al. 19 demonstrated the utility of nano zero valent (nZVI) iron for nitrate ion reduction, highlighting the potential effect of particle aggregation and catalyst.Given the various limitations of Fe(0)-based treatment systems, the successful removal of contaminants primarily depends on how well the reactivity of Fe(0) can be sustained for a longer period of time. Sustained reactivity of Fe(0) can be maintained by compensating hydrogen ions consumed during the reaction or by minimizing surface passivation by iron corrosion products. Numerous efforts to enhance performance of Fe(0) have been made in recent years, with the most involving amendment of various additive materials into Fe(0) reactions 20.
This work aims to present a new material for the chemical reduction of nitrate ion using an ilmenite ore for preparation of nano-titanium dioxide to be used as a support for the nZVI. Leaching of ilmenite in hydrochloric acid produces solution rich in both titanium and iron ions. The effects of conditions of titanium dissolution from natural ilmenite and hydrolysis were systematically studied. Thus, nZVI were prepared by subsequent reduction of iron ions in the same solution. This is to promote the performance and stability of zero valent iron in reduction of nitrate ion by supporting it on nano titania. In this way, the prepared material benefits from enhanced chemical reactivity by keeping the iron particles without agglomeration.The effect of different loadings of zero valent Fe, solution initial pH and time on the nitrate ion anion removal were investigated.
Experimental
Materials and Instruments
A representative sample of 10 kg ilmenite ore from Abu Ghalaga region, Red Sea, Egypt, was thoroughly mixed, crushed and ground to 100% –200 mesh. The chemical analysis of the ilmenite sample, was determined using X-ray fluorescence (XRF), and was given in Table 1.Chemicals of analytical grade were used for the extraction and synthesis (hydrochloric acid HCl 37% Sigma Aldrich, sulfuric acid 98%Sigma Aldrich, sodium borohydrideNaBH4 (BDH), ethanol 99%Sigma Aldrich, NaOH 99%, NaNO3 99%. Nitrate ion reagent was obtained from Hack company (USA) and used for determination of nitrate ion by spectrophotometer DR6000, Hack (USA). Double distilled water was used during the whole work.
Table 1: Chemical composition of ilmenite concentrate.
| Component | Weight, % |
| TiO2 | 41.95 |
| Fe2O3 (equivalent) | 53.83 |
| SiO2 | 2.18 |
| CaO | 0.06 |
| MgO | 0.83 |
| Al2O3 | 1.07 |
| MnO | 0.27 |
| V2O5 | 0.025 |
Chemical analysis of the ore was carried out by X-ray fluorescence (XRF) using Quant’xEnergy Dispersive-XRF Spectrometer. Chemical analysis of Fe ions in aqueous solutions was carried out using Atomic Absorption Spectrometer Model Perken-Elmer 3100. Titanium was determined spectrophotometrically by the hydrogen peroxide method at wavelength 410 nm 21. X-ray diffraction (XRD) patterns was obtained using an automated diffractometer(Philips type: PW1840), at a step size of 0.02°, scanning rate of 2° in 2θ/min, and a 2θ range from 4° to 80°. Semi-quantitative phase analysis was performed applying X’PertHighScore Plus software.
Leaching of Ilmenite Ore
For studying the different parameters affecting the dissolution of titanium and iron from ilmenite, small scale experiments were firstly applied. A 20 g ilmenite ore was inserted into a Pyrex flaskreactor provided with a reflux condenser. A 200 mL hydrochloric acid solution of a desired concentration was added and the mixture was magnetically stirred at 400 rpm under a reflux condition for a period of time from 1 to 6 hours. The system was heated to the desired temperature ( from 25 ⁰C to to 103 ⁰C) using a thermostatically controlled glycerol/water bath.The obtained slurry was filtered off using a vacuum Buchner filtration system and the residue was washed with 1% HCl and the wash liquor was mixed with the first filtrate.Ilmenite leachant rich in titanium and iron was produced
The percent of titanium or iron extraction was calculated by the followingformula:
E% =(V. C/G. P ). 100
where E% is the Ti or Fe extraction,%, C is the Ti or Fe concentration in the leachant (g/L), V is the volume of leachant (liters), G is the total mass of the ore (g) and P is the Ti or Fe concentration in the ore.
Synthesis of nZVI Loaded on Hydrated Titania Prepared from Ilmenite
A larger scale leaching experiment of the ilmenite ore was performed to produce sufficient amount of the solution rich in titanium and iron at the optimum leaching conditions. In this case a 200 g ilmenite was mixed with 2000 mL hydrochloric acid at the optimum conditions of time and temperature under reflux condition. Hydration of titanium was performed as follows :in a 5000 mL Pyrex glass beaker, 4000 mL distilled water was added and heated near boiling and then 100 mL ilmenite leachant was added and stirred magnetically. Iron powder (by the ratio 0.05 g / mL ilmenite leachant) was mixed in order to reduce almost all ferric ions to ferrous ions. Once this reduction was completed, the yellowish color of ferric chloro complex turned to colorless and the hydration started immediately. A white turbidity developed with time indicating the precipitation of the hydrated titanium dioxide (TiO2.xH2O). The mixture was left at 70 ○C for 2 hours for completion of hydration process. The produced suspension is cooled down to room temperature and the pH was adjusted to 2.0 by adding 0.1 N NaOH.
Precipitation of nZVI on hydrated titanium dioxide was performed as follows :the whole produced amount of hydrated titanium dioxide was vigorously mixed under N2 atmosphere at ambient temperature. A 150 mLof a 0.2 M aqueous solution of NaBH4was added dropwise where a black precipitate of iron was immediately appeared and the suspension turned black. The slurry was filtered off using vacuum pump and the precipitated powder was washed several times with absolute ethanol and then dried at 80 ⁰C for 24 hours.
The ferrous iron was reduced by the borohydride through the reaction shown in Eq. (1)22
Fe(H2O)62+ + 2 BH4– → Fe0 + 2 B(OH)3 + 7 H2↑ (1)
Synthesis of nZVI from Ferrous Sulfate
A 100 mL of 0.04 M aqueous solution of FeSO4.7H2O was added to a three-necked flask and vigorously mixed by a mechanical stirrer fitted with a glass rod under an atmosphere of N2 and at ambient temperature. A 100 mL of a 0.2 M aqueous solution of NaBH4was then added, dropwise, to the flask. The slurry was filtered off using vacuum pump and the precipitated powder was washed several times with absolute ethanol and then dried at 80 ⁰C for 24 hours.
Nitrate Ion Reduction
Reduction of nitrate ion was studied using nZVI alone and nZVI loaded on hydrated TiO2. Weighed amounts of the synthesized material was added into glass vials containing 10 mL of 50 mg/L sodium nitrate ion at neutral pH and the solutions were shaken for the required period of time at speed of 150 rpm at room temperature. After the desired time passed, the suspensions were filtered using vacuum pump and the solutions concentrations were measured. The nitrate ion removal efficiency were calculated according to the following formula :
% R =(Co. Ce/Co ). 100
where Co, Ce are the initial and the equilibrium nitrate ion concentration, respectively
Results and Discussion
Characterization of the Synthesized Materials
Characterization of the synthesized materials were investigated using TEM, XRD, UV-vis and EDXRF. TEM micrograph of nanotitania that was hydrolyzed from ilmenite ore leachant is presented in Fig. 1. Inspecting these micrographs revealed the formation of distinct leaf-like morphology nanocrystals with average diameter of 20 nm. It is possible to see the ‘leafy’ morphology. These ‘leaves’ dispersing evenly in solution so that it is stable more than 6 months at room temperature[23]. The particles looked dispersed homogeneously without agglomeration.
The X-ray diffraction pattern of titanium dioxide particles prepared by hydrolysis of the ilmenite leachant at 100 °C without any further heat treatment is shown in Fig. 2. Only anatase phase TiO2 was detected as the main hydrolysis product. This results agrees well with our previous work where a purified TiOCl2 is hydrolyzed at similar conditions [23].
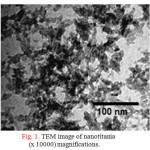 |
Figure 1: TEM image of nanotitania (x 10000) magnifications. Click here to View table |
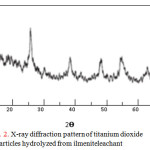 |
Figure 2: X-ray diffraction pattern of titanium dioxide particles hydrolyzed from ilmeniteleachant |
The UV-Vis optical absorption of the hydrolyzed titania and nZVI loaded titania are presented in Fig. 3. The hydrolyzed titaniashowed sharp absorption band towards the UV region (<400 nm). However, this absorption band was absent after loading the titania with nZVI. This may be due to the adhesion of nano iron particles on titania surface making isolating layers those are blocking any interaction of the light beams with TiO2 surface. This may suggest that nZVI-Titania particles will chemically react with the targeted nitrate ions without any catalytic or photocatalytic effect of the TiO2 support.
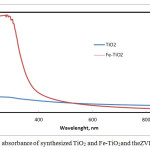 |
Figure 3: UV-Vis absorbance of synthesized TiO2 and Fe-TiO2. |
The synthesized nZVI-TiO2 was investigated using EDXRF. Figure 4 showed that the synthesized material contains about 40 % Fe and 10 % Ti.
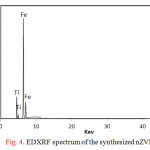 |
Figure 4: EDXRF spectrum of the synthesized nZVI-TiO2 |
Studying Leaching Conditions of Ilmenite Ore
Natural ilmenite is an inexpensive raw material contains varieties of components. The major ones are TiO2 and iron oxides. Table 1 shows an example of the chemical composition of an Egyptian low grade ilmenite ore that is used in this work. It contains a low content of TiO2 of about 42%, and a high content of total Fe of about 38%. Though, it is considered as a low grade ilmenite ore. The extraction procedures aims at maximum dissolution of titanium and iron contents in the leachant. The rich leachant will be then utilized as a source for synthesis of nZVI loaded on titanium dioxide.
It is known that the main minerals in ilmenite ore are ilmenite (FeTiO3or FeO.TiO2) which contain iron in the divalent state and titanium in the tetravalent state, and hematite (Fe2O3) which contain iron in the trivalent state[13].The ilmenite fraction in the ore is dissolved in hydrochloric acid as follows:
FeO.TiO2+ 4 HCl→ FeCl2 + TiOCl2 + H2O (2)
and the hematite fraction in the ore is dissolved in hydrochloric acid as follows:
Fe2O3+ 6HCl → 2FeCl3 + 3H2O (3)
Thus, the resulting leachant would contain Fe2+, Fe3+ and Ti4+ ions together with some metallic impurities (such as Ca2+, Mg2+, Al3+, Mn2+ and V5+) in the chloride forms and residual HCl. The following study will be concerned with optimizing the effects of HCl concentration, leaching time, and temperature for maximum extraction of titanium and iron from ilmenite ore.
Effect of Hydrochloric Acid Concentration
Figure 5 shows the effect of hydrochloric acid concentration on extraction percentage of titanium and iron from ilmeniteore at 70 ○C for 3 hours. It can be seen from this figure that hydrochloric acid was ineffective for leaching at and less than 20% where the extraction efficiencies of both metals were as low as 12% and 2% for Fe and Ti, respectively. However a sharp increase in extraction was observed at higher acid concentrations. About 94% and 90% of Fe and Ti were extracted in 30% HCl, respectively, and these values very little increased in concentrated HCl. Further studies were performed using 30% HCl.
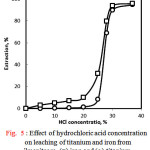 |
Figure 5: Effect of hydrochloric acid concentration on leaching of titanium and iron from ilmeniteore. (□) iron and (○) titanium. |
Effect of Leaching Time
Figure 6 shows the effect of leaching time on extraction percentage of titanium and iron from ilmenite ore at 30% hydrochloric acid and 70 ○C. It can be seen from this figure that the extraction efficiencies continuously increased with time for both metal ions up to 3 hours and the extraction was very slightly increased at longer time. About 94% and 90% of Fe and Ti, were extracted at 3 hours , respectively. These values were increased to almost 96% after 5 hours of leaching time. The next experiments were carried out at 30% HCl for 3 hours.
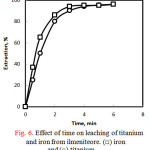 |
Figure 6: Effect of time on leaching of titanium and iron from ilmeniteore. (□) iron and (○) titanium |
Effect of Leaching Temperature
Figure 7 shows the effect of leaching temperatureon extraction percentage of titanium and iron from ilmenite ore at 30% hydrochloric acid for 5 hours. It can be seen from this figure that the temperature up to 50 ○C was in effective for the leaching where the extraction efficiencies were as low as 25% and 9.5% for Fe and Ti, respectively. However a sharp increase in extraction (about 95%) of both metal ions was observed at higher temperature such as 70 ○C. Extraction of iron was maintained at high values at higher leaching temperatures than 70 ○C, such as 90 ○C and 103 ○C but that of titanium continuously decreased reaching the value of 30%at 103 ○C. This behavior was accompanied by developing a white precipitate of probably hydrated titanium dioxide. Thus the decrease in titanium content in solution was attributed to the hydrolysis reaction of the dissolved titanyl ion (TiO2+) yielding a solid TiO2 product asfollows :
TiOCl2 + H2O → TiO2 + 2 HCl (4)
The leaching products of titanium and iron from ilmenite ore are ferric chloride (FeCl3),ferrous chloride (FeCl2) and titanyl chloride (TiOCl2). The hydrolysis of the latter to TiO2 is known to
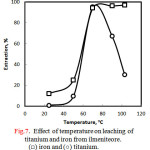 |
Figure 7: Effect of temperature on leaching of titanium and iron from ilmeniteore. (□) iron and (○) titanium. |
be favored at low acidity and high temperature. This is consistent with our previous work [26]. It is worth mentioning that at this stage the hydrolysisreactionis not favored since the main aim is to dissolve both metals.Thus, keeping the leachingtemperature at 70 ⁰C is essentialto avoid hydrolysis reaction. Any hydrolyzed products will be separated with residue by filtration and will be lost values of titanium.
Application Of the Synthesized nZVI-TiO2 in Reduction of Nitrate ion
Mechanism of Denitrification by nZVI-TiO2 and nZVI-TiO2
In acidic aqueous systems, zero-valent iron is easily oxidized to ferrous ion where the H+ acts as an electron acceptor[24]. The overall process of corrosion in an aerobic Fe˚–H2O neutral system is described by the following reaction,Eq(5). But Under aerobic conditions dissolved oxygen would play arole of the electron acceptor in the cathodic half-reaction. In this case, the primary reaction yields only OH– and not H2, Eq(6):
Fe˚ + 2H2O→Fe+2+ H2+2OH– (5)
2Fe˚ +O2+2H2O→2Fe+2+2OH– (6)
In considering the reaction products of nitrate ion reduction, Table 2 lists possible species such as NO2–, NO, N2 and NH4+ which depend primarily on the redox value (Eh0). In view of redox value, the N2 is relatively more difficult to produce (Eh0 = 1.241 V), and the next one is NO (Eh0= 0.952 V).Though the NO2– might occur as one of the reaction products (Eh0 = 0.843 V), it easily becomes converted into NH4+ (Eh0 = 0.89 V). It appears that NH4+ might dominate among these reaction products. As discussed later, the end reduction product was accounted for mostly by NH4+.
![Table 2: Equilibrium constants for redox half-cell reactions of nitrate ion[49]](http://www.orientjchem.org/wp-content/uploads/2016/08/Vol32No4_Stud_Moha_tab2-150x150.jpg) |
Table 2: Equilibrium constants for redox half-cell reactions of nitrate ion |
A schematic diagram in Fig. 8 shows the possible nitrate ion reduction mechanism. Ilmenite leachant is rich in both titanium in the tetravalent state (TiO2+) and iron in both divalent (Fe2+) and trivalent (Fe3+) states. All these species would be in the chloride forms. It is important to note that no hydration of the TiO2+totheTiO2species will take place unless all the iron is in the Fe2+state. Therefore, addition of the calculated amount of iron powder reduces the Fe3+ portion to the Fe2+ state. At this stage the (TiO2+) species can be easily converted to the white hydrated titania (TiO2) by adding the reduced leachant to boiling water with the desired volume ratio. The pH of the suspension is then adjusted to around 2 to avoid the re-oxidation of iron.Then, the whole Fe2+in the suspension is reduced to the zero valent state by addition of calculated amount of NaBH4. The precipitated iron sticks to the hydrated TiO2 and the color is changed to black. The formed nZVI-TiO2 held in suspension for about 2 hours without settling. This product is very active so that if does not separated from the solution it react with water forming the brownish Fe(OH)3. To avoid this, the nZVI-TiO2 is quickly separated and thoroughly washed with ethanol and dried. When nZVI-TiO2is contacted with NO3– in water, it will reduce it mostly to NH4+.
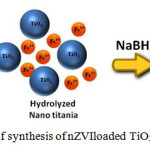 |
Figure 8: Schematic diagram of synthesis of nZVIloaded TiO2 and nitrate ion reduction |
Effect of Reducing Material (nZVI or nZVI-TiO2)
Nitrate ion reduction was firstly tested using either nZVI or nZVI loaded on TiO2 surface (nZVI-TiO2). Fig. 9 shows a bar graph comparing the nitrate ion reduction using 0.5g, 1.0g and 2g of each material at pH 7 for 3 hours. It can be seen that the efficiency of nZVI-TiO2 is always superior to that of nZVI.
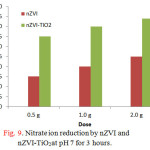 |
Figure 9: Nitrate ion reduction by nZVI and nZVI-TiO2at pH 7 for 3 hours. |
Effect of shaking time
The effect of shaking time on reduction of nitrate ion using 0.5 and 1 g nZVI-TiO2 at pH 7 is shown in Fig 10. For the two doses, the removal efficiency % of nitrate ion continuously increased with time till about 120 min and then slowly increased. These values reached around 35% and 40% after 180 min for 0.5 and 1.0 g nZVI-TiO2, respectively.
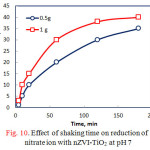 |
Figure 10: Effect of shaking time on reduction of nitrate ion with nZVI-TiO2 at pH 7 |
Effect of solution pH
The effect of solution pH ranging from 1 to 7 on the removal efficiency of nitrate ionafter 3 hours was performed using two doses of nZVI-TiO2(0.5g and 1.0g) and the results were presented in Fig. 11. The results revealed that the nitrate ion removal efficiency was gradually decreasing with pH in the acidic and alkaline region. About 50% and 60% of nitrate ion could be removed at pH = 1 and these values decreased to 30% and 35% at pH = 9 using 0.5g and 1.0 g nZVI-TiO2 doses, respectively. It is worth mentioning that the performance of Fe(0) system in terms of iron reactivity and permeability of Fe(0) bed is negatively affected at above-neutral medium as passivating minerals such as iron oxides, iron sulfides, and carbonate minerals begin to develop on Fe(0) surface.
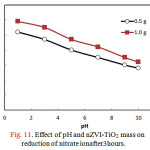 |
Figure 11: Effect of pH and nZVI-TiO2 mass on reduction of nitrate ion after 3 hours. |
Conclusion
In this work, we have succeeded in preparation of a new material composed of hydrated titanium dioxide (TiO2) loaded with nano-zero valent iron, from the inexpensive Egyptian ilmenite ore. The ilmenite ore was found suitable for leaching with hydrochloric acid to produce a solution rich in both titanium and iron ions, Using 30% hydrochloric acid at 70 ○C and 5 hours, most of iron and titanium in the ilmenite ore were dissolved. Nano hydrated titania was firstly produced by hydrolysis of the ilmenite leachant and nZVI was subsequently precipitated on its surface by reduction with sodium borohydride in nitrogen atmosphere. The synthesized nZVI-TiO2 showed better performance in nitrate reduction than the nZVI alone, where the latter is easily agglomerates and its activity decreases.
References
- Wolfe, A.H.;Patz, J.A.Ambio2002, 31, 120–125.
CrossRef - Rao, P.;Mak, M.S.H.; Liu, T.; Lai, K.C.K. ; Lo, I.M.C., Chemosphere2009, 75, 156–162.
CrossRef - Thomson, T.S. Bull. Environ. Contam.Toxicol.2001, 66, 64–70.
CrossRef - Bae, B.U.Y.H. ; Jung, Y.H. ; Han, W.W. ; Shin, H.S.Water Res.2002, 36, 3330–3340.
CrossRef - Schoeman, J.J. ;Steyn, A. Desalination2003,155, 15–26.
CrossRef - Fernandez-Nava, Y. ; Maranon, E.;Soons, J. ;Castrill, L.Bioresour.Technol.2008, 99, 7976–7981.
CrossRef - Bhatnagar, A.; Sillanpaa, M.Chem. Eng. J.2011, 168, 493–504.
CrossRef - Hwang, Y.H.; Kim, D.-G. ; Shin, H.-S.J. Hazard. Mater.2011, 185, 1513–1521.
CrossRef - Zhang, Y.; Li, Y.; Hu, L.;Zheng, X. Chem. Eng. J.2011, 171, 526–531
CrossRef - Akhgar, B.N.;Pazouki, M.;Ranjbar, M.;Hosseinnia, A.;Salarian, R.Chem. Eng. Res. Des., 2012, 90, 220–228.
CrossRef - Manhique, A.J.;Focke, W.W.;Madivate, C.Hydrometallurgy2011, 109, 230–236.
CrossRef - Zhang, l.; Hu, H.; Liao, Z.; Chen, Q.; Tan, J. Hydrometallurgy2011, 107, 40–47.
CrossRef - Mahmoud M.H.H.;Afifi A.A.I.; Ibrahim I.A.Hydrometallurgy, 2004, 73, 99-109.
CrossRef - Mahmoud, M.H.H.Sep. Purif. Technol.2012, 84, 63–71.
CrossRef - Mahmoud, M.H.H.; Ismail, A. A.; Sanad, M. M. S. Chem. Eng. J.2012, 187, 96-103.
CrossRef - Mostafa, N.Y.;Zaki, Z.I.;Heiba, Z.K.; J. Magn. Mater.2013, 329, 71–76.
CrossRef - Gillham, R.W.;Ohannesin, S.F.Ground Water1994, 32, 958–967.
CrossRef - Liu, C.-C.; Tseng, D.-H.; Wang, C.-Y.J. Hazard. Mater.2009, 136, 706–713.
CrossRef - Ryu, A.;Jeong, S.-W.; Jang, A.; Choi, H.Appl. Catal. B2011, 105, 128–135.
CrossRef - Cho, D.W.;Chon, C.-M.; Jeon, B. H.; Kim, Y.; Khan, M.A.; Song, H.;Chemosphere2010, 81, 611–616.
CrossRef - Vogel, A.I.A Textbook of Quantitative Inorganic Analysis, 1978, 4th ed. Longman, London.
- Lien, H.L.; Zhang, W.X. ColloidSurf. A-Physicochem.Eng. Asp.2001, 191, 97–105.
CrossRef - Mostafa, N. Y.; Mahmoud, M.H.H.; Heiba, Z.K. Hydrometallurgy2013, 139, 88-94.
CrossRef - Ziajahromi1, S.; Zand, A. D.; Khanizadeh, M. International Conference on Applied Life Sciences (ICALS2012)2012, Turkey, Septemsion

This work is licensed under a Creative Commons Attribution 4.0 International License.









