A Study of Fe3O4@ Zigzag, @Armchair and @Chiral Swcnts and α and γ Cyclodextrins
Somayeh Khosravi
Department of Chemistry, Science and research Branch, Islamic Azad University, Tehran, Iran.
Corresponding Author Email: S.khosravi1986@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/320421
Article Received on :
Article Accepted on :
Article Published : 08 Aug 2016
Fe3O4 is used as a catalyst in the Haber process and in the water gas shift reaction. In this work we have investigated the physical and chemical properties of Fe3O4@ Zigzag, @Armchair and @Chiral SWCNTs compare to Fe3O4@ -Cyclodextrin in view point of chemical reaction and sensitizes. The electrical properties such as NMR Shielding, electron densities, energy densities , potential energy densities, ELF, LOL, ellipticity of electron density , eta index and ECP for Fe3O4@ -Cyclodextrin shell and Fe3O4@ Zigzag, @Armchair and @Chiral SWCNTs have been calculated for the simulations. Our Calculation indicate that the Fe3O4 @ (7, 7) and Fe3O4 @ (10,5) and Fe3O4 @ (9, 0) have physical and chemical properties close to Fe3O4@ -Cyclodextrin and as well as the Fe3O4 @ (8,8) and Fe3O4 @ (11,6) and Fe3O4 @ (10, 0) are close to Fe3O4@ -Cyclodextrin.
KEYWORDS:Fe3O4; Nano-Particles; electron density; SWCNTs; Cyclodextrin (α,γ)
Download this article as:| Copy the following to cite this article: Khosravi S. A Study of Fe3O4@ Zigzag, @Armchair and @Chiral Swcnts and α&γ Cyclodextrins. Orient J Chem 2016;32(4). |
| Copy the following to cite this URL: Khosravi S. A Study of Fe3O4@ Zigzag, @Armchair and @Chiral Swcnts and α&γ Cyclodextrins. Orient J Chem 2016;32(4). Available from: http://www.orientjchem.org/?p=19898 |
Introduction
Fe3O4 nanoparticles (MNPs) are one of the most intensively studied magnetic nanoparticles and can be applied in a variety of areas, ranging from drug delivery1 and bio-sensing2, dynamic sealing 3, and cell labeling4 to magnetic resonance imaging5.
Fe3O4 is ferromagnetic with a curie temperature of 858 K and The ferromagnetism of Fe3O4 arises because the electron spins of the FeII and FeIII ions in the octahedral sites are coupled and the spins of the FeIII ions in the tetrahedral sites are coupled but anti-parallel to the former6,7.
Fe3O4 is used as a catalyst in the Haber process and in the water gas shift reaction 7, 8.
Moreover, MNPs are well suited for target capturing, enrichment, and isolation 9. Accordingly, they can be used for isolating cells 10 and bacteria 11 and for removing environmental toxins such as heavy metals and chemical waste 12.
The latter uses an HTS (high temperature shift catalyst) of iron oxide stabilized by chromium oxide 6-8. This iron-chrome catalyst is reduced at reactor start up to generate Fe3O4 from α-Fe2O3 and Cr2O3 to CrO3 5. Fe3O4 is an electrical conductor with conductivity significantly higher than Fe2O3, and this is ascribed to electron exchange between the FeII and FeIII centers 6-9.
Magnetite (Fe3O4) is the earliest discovered magnet which crystallizes in the inverse cubic spinel structure. Each cubic spinel cell contains eight interpenetrating oxygen and the tetrahedral sites, occupied by one-third of the iron atoms, form a diamond structure. The remaining Fe atoms are located at the octahedral sites with the nearest-neighbor atoms lined up as strings along six different [110] directions. In other words Fe3O4 consists of a cubic close packed array of oxide ions where all of the Fe2+ ions occupy half of the octahedral sites and the Fe3+ are split evenly across the remaining octahedral sites and the tetrahedral sites 6, 13, and 14.
Nano sized magnetic particles are considered potential adsorbents for aqueous heavy metals due to their high surface area and the unique advantage of easy separation under external magnetic fields 15–19. To further facilitate the adsorption affinity, surface modification, including physical coating and covalent binding, has often been explored to enable specific metal complexation20–25. For example, covalent attachment of carboxylic groups to the surface of magnetic nanoparticles was achieved by reacting hematite with 11-undecanoic acid, and the resulting
material showed marked ability in adsorbing Cd (II) 20. The chitosan-coated Fe3O4 nanoparticles were reported to be efficient for the removal of Cu (II) ions 22. Humic-acid-coated Fe3O4 nanoparticles prepared by the co-precipitation method were found to effectively sorb Hg (II), Pb (II), Cd (II), and Cu (II) from water 23. The thiol groups on the surfaces of Di-Mercapto-succinic acid-coated Fe3O4 nanoparticles also acted as binding ligands for Hg, Ag, Pb, Cd, and Tl metal ions24. Recently, a magnetic nano-adsorbent prepared by coating a magnetite core with chitosan, followed by carboxylation with a keto-glutamic acid, was used to remove Cu (II) ions from aqueous solution 25.
SiO2 is stable under acidic conditions and inert to redox reactions, as compared with the organic coating materials, and hence functions as an ideal shell composite to protect the inner magnetite core26–28. Silica-coated core–shell magnetite nanoparticles, i.e., Fe3O4@SiO2, have recently been investigated for potential biomedical applications 27, 28. Additionally, the SiO2 coating
shell has an abundance of surface hydroxyl groups, which offers ease of functionalization of magnetite nanoparticles.
Cyclodextrins, cyclic oligosaccharides obtained from the degradation of starch by Bacillus
macerans, were first isolated in the late nineteenth century 29. Their ability to form inclusion complexes with suitable organic molecules was discovered soon thereafter30. With the development of the field of Supramolecular Chemistry, their complexation properties have been extensively studied31 Applications for cyclodextrins and their derivatives are sought in various areas of chemistry, including the sensing of organic molecules32.
Carbon nanotube (CNT) is a representative nano-material. CNT is a cylindrically shaped carbon material with a nano-metric-level diameter33.Its structure, which is in the form of a hexagonal mesh, resembles a graphite sheet and it carries a carbon atom located on the vertex of each mesh. The sheet has rolled and its two edges have connected seamlessly33, 34.
Although it is a commonplace material using in pencil leads, its unique structure causes it to present characteristics that had not found with any other materials. CNT can be classified into single-wall CNT, double-wall CNT and multi-wall CNT according to the number of layers of the rolled graphite. The type attracting most attention is the single-wall CNT, which has a diameter deserving the name of “nanotube” of 0.4 to 2 nanometers. The length is usually in the order of microns, but single-wall CNT with a length in the order of centimeters has recently released.
SWCNTs have considered as the leading candidate for nano-device applications because of their one-dimensional electronic bond structure, molecular size, and biocompatibility, controllable property of conducting electrical current and reversible response to biological reagents hence SWCNTs make possible bonding to polymers and biological systems such as DNA and carbohydrates.
Theoretical Background
Electron Density Profile Models
The electron density has been defined as
![]()
Where is occupation number of orbital (i), is orbital wave function, c is basis function and C is coefficient matrix, the element of ith row jth column corresponds to the expansion coefficient of orbital j respect to basis function i. Atomic unit for electron density can be explicitly written as e/Bohr3.
![]()
The positive and negative value of this function correspond to electron density is locally depleted and locally concentrated respectively. The relationships between ρ and valence shell electron pair repulsion (VSEPR) model, chemical bond type, electron localization and chemical reactivity have been built by Bader38.
Hamiltonian Kinetic Energy Density K(R)
The kinetic energy density is not uniquely defined, since the expected value of kinetic energy operator
![]()
can be recovered by integrating kinetic energy density from alternative definitions. One of commonly used definition is:
![]()
Relative to K(r), the local kinetic energy definition given below guarantee positivizes everywhere; hence the physical meaning is clearer and is more commonly used.
The Lagrangian kinetic energy density, “G(r)” is also known as positive definite kinetic energy density.
![]()
K(r) and G(r) are directly related by Laplacian of electron density
![]()
Electron Localization Function (ELF)
Becke and Edgecombe noted that spherically averaged likespin conditional pair probability has direct correlation with the Fermi hole and then suggested electron localization function (ELF).

Savin et al. have reinterpreted ELF in the view of kinetic energy, 40 which makes ELF also meaningful for Kohn-Sham DFT wave-function or even post-HF wave-function. They indicated that D(r) reveals the excess kinetic energy density caused by Pauli repulsion, while D0(r) can be considered as Thomas-Fermi kinetic energy density 41.
Localized orbital locator (LOL) is another function for locating high localization regions likewise ELF, defined by Schmider and Becke in the paper42, 43.
![]()
where
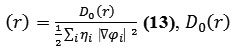
for spin-polarized system and close-shell system are defined in the same way as in ELF43.
This work has been studied based some previous works44-167 and has compared with the experimental data.
Result and Discussion
This study mainly focuses on the magnetic properties of Fe3O4 in a non-bonded system with Cyclodextrin ( . Part of the systems including Fe3O4@ -Cyclodextrin nanoparticles have been considered in this work. It has been exhibited that the Fe3O4 @ (7, 7) and Fe3O4 @ (10, 5) and Fe3O4 @ (9, 0) have physical and chemical properties close to Fe3O4@ -Cyclodextrin and as well as the Fe3O4 @ (8, 8) and Fe3O4 @ (11, 6) and Fe3O4 @ (10, 0) are close to Fe3O4@ -Cyclodextrin.
The data are shown in tables 1-6 and figures 1-7 for a non-bonded interaction of the Fe3O4 with Gamma CD and various nanotubes.
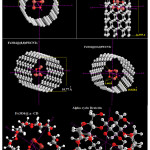 |
Figure 1: Optimized Fe3O4@various nanotubes and cyclodexterin Click here to View Figure |
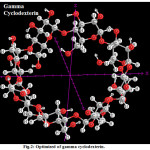 |
Figure 2: Optimized of gamma cyclodexterin Click here to View Figure |
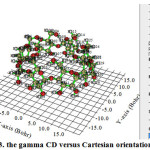 |
Figure 3: The gamma CD versus Cartesian orientation Click here to View Figure |
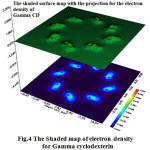 |
Figure 4: The Shaded map of electron density for Gamma cyclodexterin Click here to View Figure |
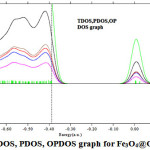 |
Figure 5: TDOS, PDOS, OPDOS graph for Fe3O4@GammaCD Click here to View Figure |
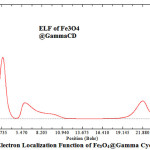 |
Figure 6: Electron Localization Function of Fe3O4@Gamma Cyclodexterin Click here to View Figure |
 |
Figure 7: Localized orbital Locator for Fe3O4@ (7,7)SWCNTs Click here to View Figure |
This work has been modeled with QM/MM method and the calculations are carried out with the Advanced DFT methods. Gaussian 2009 and a HyperChem professional release 7.01 programs are used for the additional calculations. For non-covalent interactions between Fe3O4 and Cyclodextrins, the B3LYP method is unable to describe van der Waals by medium-range interactions. Therefore, the ONIOM methods including 3 levels of 1-high calculation (H), 2-medium calculation (M), and 3-low calculation (L) have been performed in our study for calculating the non-bonded interactions between Fe3O4 and Cyclodextrins.
The ab-initio and DFT methods are used for the model system of the ONIOM layers and the semi empirical methods of pm3mm (including pseudo=lanl2) and Pm6 are used for the medium and low layers, respectively.
B3LYP and the most other popular and widely used functional are insufficient to illustrate the exchange and correlation energy for distant non-bonded medium-range systems correctly. Moreover, some recent studies have shown that inaccuracy for the medium-range exchange energies leads to large systematic errors in the prediction of molecular properties 168-170.
Geometry optimizations and electronic structure calculations have been carried out using the m06 (DFT) functional. This approach is based on an iterative solution of the Kohn-Sham equation 171 of the density functional theory in a plane-wave set with the projector-augmented wave pseudo-potentials. The Perdew-Burke-Ernzerhof (PBE)172 exchange-correlation (XC) functional of the generalized gradient approximation (GGA) is also used. The optimizations of the lattice constants and the atomic coordinates are made by the minimization of the total energy.
The charge transfer and electrostatic potential-derived charge were also calculated using the Merz-Kollman-Singh chelp or chelpG173-176 the charge calculation methods based on molecular electrostatic potential (MESP) fitting are not well-suited for treating larger systems whereas some of the innermost atoms are located far away from the points at which the MESP is computed. In such a condition, variations of the innermost atomic charges will not head towards a significant change of the MESP outside of the molecule, meaning that the accurate values for the innermost atomic charges are not well-determined by MESP outside the molecule . The representative atomic charges for molecules should be computed as average values over several molecular conformations.
A detailed overview of the effects of the basis set and the Hamiltonian on the charge distribution can be found in references175-177. The charge density profiles in this study has been extracted from first-principles calculation through an averaging process as described in reference 175-177. The interaction energy for capacitor was calculated in all items according to the equation as follows:
![]()
Where the “ ” is the stability energy.
The electron density (Both of Gradient norm & Laplacian), value of orbital wave-function, electron spin density, electrostatic potential from nuclear atomic charges, electron localization function (ELF), localized orbital locator (LOL defined by Becke & Tsirelson), total electrostatic potential (ESP), as well as the exchange-correlation density, correlation hole and correlation factor, and the average local ionization energy using the Multifunctional Wave-function analyzer have also been calculated in this study 35-37. The contour line map was also drawn using the Multiwfn software 35, 36. The solid lines indicate positive regions, while the dash lines indicate negative regions. The contour line corresponding to VdW surface (electron density=0.001 a.u., which is defined by R. F. W Bader) is plotted in this study. This is specifically useful to analyze distribution of electrostatic potential on VdW surface. Such a contour line has also been plotted in gradient line and vector field map by the same option. The relief map was used to present the height value at every point. Shaded surface map and shaded surface map with projection are used in our representation of height value at each situation 35-37.
Electrical properties can be obtained from changes in the non-bonded interaction situations. Electron densities, energy densities , Potential energy densities, ELF, LOL, Ellipticity of electron density , eta index and ECP for Fe3O4– Cyclodextrin ( shell and Fe3O4 @ (7, 7), Fe3O4 @ (10, 5), Fe3O4 @ (9, 0) , Fe3O4 @ (8, 8), Fe3O4 @ (11, 6) and Fe3O4 @ (10, 0) were calculated for each of simulation (Table1-6).
Table 1: All Electron Densities of non-bonded interactions for Fe3O4-Cyclodextrin (γ)
| Atomnumber | Density of all electron | Density of alpha | Density of Beta( | Spin Density | Lagrangian kinetic [G(r)]energy( | Hamiltonian kinetic [K(r)]energy( |
| Fe(1) | 0.14 | 0.07 | 0.07 | 0.0 | 0.22 | 0.32 |
| Fe(2) | 0.26 | 0.13 | 0.13 | 0.0 | 0.26 | 0.56 |
| Fe(3) | 0.32 | 0.16 | 0.16 | 0.0 | 0.11 | 0.24 |
| O(1) | 0.24 | 0.12 | 0.12 | 0.0 | 0.28 | -0.12 |
| O(2) | 0.18 | 0.09 | 0.09 | 0.0 | 0.31 | -0.22 |
| O(3) | 0.40 | 0.2 | 0.2 | 0.0 | 0.27 | -0.11 |
| O(4) | 0.30 | 0.15 | 0.15 | 0.0 | 0.11 | -0.22 |
Table 2: Laplacian, ELF, LOL and Local information entropy of non-bonded interactions for Cyclodextrin (γ)
| Atomnumber | Laplacian of electron density( | Electron localization function ELF ( | Localized orbital locator (LOL) ( | Local information entropy( | Reduced density gradient(RDG)( | Average local ionization energy |
| Fe(1) | -0.15 | 0.60 | 0.18 | 0.17 | 0.39 | 0.32 |
| Fe(2) | -0.16 | 0.42 | 0.28 | 0.19 | 0.41 | 0.39 |
| Fe(3) | -0.20 | 0.32 | 0.18 | 0.41 | 0.52 | 0.50 |
| O(1) | 0.38 | 0.32 | 0.24 | 0.18 | 0.44 | 0.34 |
| O(2) | 0.41 | 0.22 | 0.15 | 0.29 | 0.16 | 0.16 |
| O(3) | 0.35 | 0.26 | 0.34 | 0.31 | 0.47 | 0.83 |
| O(4) | 0.34 | 0.33 | 0.22 | 0.25 | 0.24 | 0.20 |
Table 3: Lambada2, Wave function value, Ellipticity of electron density and Eta index of non-bonded interactions for Cyclodextrin ( γ)
| Atomnumber | Lambada2( | Wave function value | Ellipticity of electron density | Eta index | ESP from nuclear charge ( | ESP from electron charge ( |
| Fe(1) | -0.12 | 0.41 | 0.40 | -3.15 | 0.10 | -0.41 |
| Fe(2) | -0.14 | 0.89 | 0.40 | -3.65 | 0.10 | -0.41 |
| Fe(3) | -0.14 | 0.62 | 0.33 | 1.77 | 0.10 | -0.42 |
| O(1) | 0.35 | 0.31 | -0.44 | 1.47 | 0.11 | -0.43 |
| O(2) | 0.15 | -0.41 | -0.27 | 0.90 | 0.11 | -0.41 |
| O(3) | 0.31 | 0.35 | -0.43 | 0.68 | 0.11 | -0.42 |
| O(4) | 0.28 | -0.38 | -0.27 | 0.66 | 0.15 | -0.41 |
Table 4: All Electron Densities of non-bonded interactions for Fe3O4 @ (11, 6) SWCNTs
| Atomnumber | Density of all electron | Density of alpha | Density of Beta | Spin Density | Lagrangian kinetic [G(r)]energy | Hamiltonian kinetic [K(r)]energy | EnergyDensity[E(r)] |
| Fe(1) | 0.42 | 0.21 | 0.21 | 0.0 | 0.51 | 0.55 | -0.41 |
| Fe(2) | 0.62 | 0.31 | 0.31 | 0.0 | 0.25 | 0.24 | -0.23 |
| Fe(3) | 0.78 | 0.39 | 0.39 | 0.0 | 0.22 | 0.23 | -0.21 |
Table 5: Laplacian, ELF, LOL and Local information entropy of non-bonded interactions for Fe3O4@ (8,8) SWCNTs
| Atomnumber | Laplacian of electron density | Electron localization function (ELF) ( | Localized orbital locator (LOL) | Local information entropy | Reduced density gradient(RDG)( | Average local ionization energy | ESP from electron charge ( |
| Fe(1) | -0.08 | 0.31 | 0.22 | 0.20 | 0.18 | 0.49 | -0.68 |
| Fe(2) | -0.08 | 0.30 | 0.12 | 0.14 | 0.35 | 0.51 | -0.67 |
| Fe(3) | -0.09 | 0.48 | 0.12 | 0.21 | 0.22 | 0.33 | -0.68 |
Table 6: Lambada2, Wave function value, Ellipticity of electron density and Eta index of non-bonded interactions for Fe3O4@(10,0)SWCNTs
| Atom(number) | Lambada2( | Wave function value | Ellipticity of electron density | Eta index |
| Fe(1) | -0.31 | 0.14 | 0.98 | 1.2 |
| Fe(2) | -0.54 | 0.15 | 0.92 | 2.1 |
| Fe(3) | 0.23 | -0.34 | 0.90 | 0.5 |
We believe that this research has a great potential for the development of new multifunctional catalysts with high reactivity and selectivity. Another interesting development is using the SWCNTs on magnetic nanoparticles that enable effective removal of transition metal based catalysts from important pharmaceutical products in drug synthesis. This approach should find relevant industrial applications in biopharmaceutical, food additives, fragrances and other sectors.
References
- Veiseh, O.; Gunn, J.W.; Zhang, M. Advanced Drug Delivery Reviews, 2010. 62, 3, 284–304.
- Yang, L.; Ren, X.; Tang, F.; Zhang, L.; Biosensors and Bioelectronics, 2009, 25, 4, 889–895.
- Ripka, P, Journal of Magnetism and Magnetic Materials, 2000, 215, 735–739.
- Wilhelm, C.; Gazeau, F. Biomaterials, 2008, 29( 22), 3161–3174.
- Jain, T.K.; Foy, S.P.; Erokwu, B.; Dimitrijevic, S.; Flask, C.A.; Labhasetwar, V. Biomaterials, 2009,30(35), 6748–6756.
- Ma, Ming; Zhang, Yu; Guo, Zhir4.ui; GU, Ning, Nanoscale Research Letters, 2013, 8 (1), 16.
- Massart, R.; IEEE transactions on magnetics, 1981,17(2), 1247–1248
- Sunggyu Lee Encyclopedia of Chemical Processing CRC Press, 2006, ISBN 0-8247-5563-4
- Agrawal, A.; Sathe, T.; Nie, S.; Journal of Agricultural and Food Chemistry, 2007, 55(10), 3778–3782.
- Liang, X.; Xu, K.; Xu, J.; Chen, W.; Shen, H. Liu, J. Journalof Magnetism and Magnetic Materials, 2009,32(12), 1885–1888.
- Shan, Z.; Wu, Q.; Wang, X. Analytical Biochemistry, 2010, 398, 1, 120–122.
- Shen, Y. F.; Tang, J.; Nie, Z. H. ; Wang, Y. D.; Ren, Y.; Zuo, L.Separation and Purification Technology, 2009, 68, 3, 312–319.
- Valter, Ström; Richard T.; Olsson, K. V. Rao. J. Mater. Chem, 2010, 20, 4168-4175
- Mei Fang, ValterStröm, Richard T. Olsson, LyubovBelova, K. V. Rao, Rapid mixing: Appl. Phys. Lett.2011, 99, 222501
- Shin, S.; Jang, J.; Chem. Commun.2007, 41, 4230.
- Oliveira, L.C.A.;Petkowicz,D.I.; Smaniotto, A.; Pergher, S.B.C.; Water Res.2004, 38 3699.
- Hu, J.; Lo, M.C.; Chen, G.H.; Water Sci. Technol.2004, 50,139.
- Hu, J.; Chen, G.; Lo, I.M.C. Water Res. 2005, 39, 4528.
- Yavuz, C.T.; Mayo, J.T.; Yu, W.W.; Prakash, A.; Falkner, J.C.; Yean, S.; Cong, L.; Shipley, H.J.; Kan, A. ; Tomson, M. ; Natelson, D.; Colvin, V.L.; Science,2006, 314,964.
- Hai, B.; Wu, J.; Chen, X. ; Protasiewicz, J.D.; Scherson, D.A. ; Langmuir,2005, 21 3104.
- Hu, J.; Lo, M.C.; Chen, G.H.; Sep. Purif. Technol.2007, 58, 76.
- Chang, Y.C.; Chen, D.H.; J. Colloid Interface Sci.2005, 283,446.
- Liu, J.F.; Zhao, Z.S.; Jiang, G.B.; Environ. Sci. Technol.2008, 42, 6949.
- Yantasee, W.; Warner, C.L.; Sangvanich, T.; Addleman, R.S.; Carter, T.G. ; Wiacek, R.J.; Fryxell, G.E.; Timchalk, C.; Warner, M.G.; Environ. Sci. Technol.2007, 41, 5114.
- Zhou, Y.T.; Nie, H.L.; Branford-White, C.; He, Z.Y. J. Colloid Interface Sci.2009, 330 29.
- Liu, Q.; Xu, Z.; Finch, J.A.; Egerton, R. Chem. Mater. 1998,10 , 3936.
- Lu, C.W.; Hung, Y.; Hsiao, J.K.; Yao, M.; Chung, T.H.; Lin, Y.S.; Wu, S.H.; Hsu, S.C.; Liu, H.M.; Mou, C.Y.; Yang, C.S.; Huang, D.M.; Chen, Y.C.; Nano Lett.2007, 7 , 149.
- Ashtari, P. He, X.X.; Wang, K.; Gong, P.; Talanta ,2005,67 ,548.
- Villiers, A. Compt. Rend. 1891, 112, 536-539.
- Schardinger, F.; Zentralbl. Bakteriol. Abt. II 1911, 29, 188-197; (b) Freudenberg, K.; Schaaf, E. Dumpert, G.; Ploetz, T. Naturwissenschaften, 1937, 27, 850-853
- Wenz, G. Angew. Chem. Int. Ed. Engl. 1994, 33, 803-822
- Ueno, A. Supramol. Sci. 1996, 3, 31-36
- Skidmore-Roth, L.; (Ed.). Mosby’s Nursing Drug Reference,2010, (23rd ed.). St. Louis, MO: Mosby Elsevier
- Paganelli, CV.; Solomon AK .J Gen Physiol, 1957, 41, 259-277
- Lu, T.; Chen, F. ActaChim. Sinica,2011, 69, 2393-2406.
- Lu, T.; Chen, F.; J. Mol. Graph. Model.2012, 38: 314-323.
- Lu, T.; Chen, F.; J. Comp. Chem. 2012,33 580-592.
- Bader, R.F.W. Atoms in Molecule: A quantum Theory (Oxford Univ. press, Oxford, (1990).
- Becke and Edgecombe J. Chem. Phys., 1990, 92, 5397.
- Savin,A.; Angew. Chem. Int. Ed.Engl., 1992, 31, 187.
- Tsirelson, V.G.; Abramov.Yu.A. Chem. Phys. Lett, 2002, 351, 142–148.
- Schmider, H.L.; Becke, A.D. J MolStruct (Theochem),2000,527:5150.
- Jacobsen Can. J. Chem., 2008, 86(7), 695.
- Jamali,Z.; Monajjemi,M.; J. Comput. Theor. Nanosci.2016, 13, 643–651.
- Mirzaei ,R.; Ziglari ,A.; Elsagh,A.; Esmkhani,R.; Monajjemi, M.; J. Comput. Theor. Nanosci.2016, 13, 899–908.
- Monajjemi, M.; Lee, V.S.; Khaleghian, M.; B. Honarparvar, B.; F. Mollaamin, F. J. Phys.Chem C. 2010, 114, 15315
- Monajjemi, M.Struct Chem.2012, 23,551–580
- Monajjemi, M.; Chegini, H.; Mollaamin, F.; Farahani, P. Fullerenes, Nanotubes, and Carbon Nanostructures. 2011,19, 469–482
- Monajjemi, M .; Afsharnezhad ,S.; Jaafari , M.R.; Abdolahi ,T.; Nikosade ,A.; Monajemi ,H.; Russian Journal of physical chemistry A, 2007,2,1956-1963
- Monajjemi, M.;Baei, M.T.;Mollaamin, F. Russian Journal of Inorganic Chemistry. 2008, 53 (9),1430-1437
- Monajjemi, M.; Rajaeian, E.; Mollaamin, F.; Naderi, F.; Saki, S. Physics and Chemistry of Liquids. 2008, 46 (3), 299-306
- Monajjemi, M.; Boggs, J.E. J. Phys. Chem. A, 2013,117,1670 −1684
- Mollaamin, F.; Monajjemi, M, Journal of Computational and Theoretical Nanoscience. 2012, 9 (4) 597-601
- Monajjemi, M.; Khaleghian, M, Journal of Cluster Science. 2011, 22(4),673-692318
- Nafisi, S.;Monajemi, M.;Ebrahimi, S. Journal of Molecular Structure. 2004,705 (3)35-39
- Fazaeli, R.; Monajjemi, M.; Ataherian, F.; Zare, K. Journal of Molecular Structure: THEOCHEM.2002, 581 (1), 51-58
- Monajjemi, M.; Razavian, M.H.; Mollaamin,F.; Naderi,F.; Honarparvar,B.; Russian Journal of Physical Chemistry A , 2008 , 82 (13), 2277-2285
- Monajjemi, M.; SeyedHosseini, M.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures. 2013, 21, 381–393
- Monajjemi, M.; Faham, R.; Mollaamin, F.Fullerenes, Nanotubes, and Carbon Nanostructures,2012 20, 163–169
- Mollaamin, F.; Najafi, F.; Khaleghian, M.; KhaliliHadad, B.; Monajjemi, M. Fullerenes, Nanotubes, and Carbon Nanostructures, 201119, 653–667
- Mollaamin, F.; Baei, MT.; Monajjemi, M.; Zhiani, R.; Honarparvar, B.;Russian Journal of Physical Chemistry A, Focus on Chemistry, 2008, 82 (13), 2354-2361
- Monajjemi, M. Chemical Physics. 2013, 425, 29-45
- Monajjemi, M.; Heshmat, M.; Aghaei, H.; Ahmadi, R.; Zare, K. Bulletin of the Chemical Society of Ethiopia, 2007,21 (1)
- Monajjemi, M.;Honarparvar, B. H. ; Haeri, H. ; Heshmat ,M.; Russian Journal of Physical Chemistry C. 2006, 80(1):S40-S44
- Monajjemi, M.; Ketabi, S.; Amiri, A. Russian Journal of Physical Chemistry, 2006, 80 (1), S55-S62
- Yahyaei, H.; Monajjemi, M.; Aghaie, H.; K. Zare, K. Journal of Computational and Theoretical Nanoscience. 2013, 10(10), 2332–2341
- Mollaamin, F.; Gharibe, S.; Monajjemi, M. Int. J. Phy. Sci, 2011, 6, 1496-1500
- Monajjemi, M.; Ghiasi, R.; SeyedSadjadi, M.A.Applied Organometallic Chemistry,2003,17(8), 635–640
- Monajjemi, M.; Wayne Jr, Robert. Boggs, J.E. Chemical Physics.2014, 433, 1-11
- Monajjemi, M.; Sobhanmanesh, A.; Mollaamin, F.Fullerenes, Nanotubes, and Carbon Nanostructures, 2013, 21 47–63
- Monajjemi, M.; Mollaamin, F. Journal of Computational and Theoretical Nanoscience, 2012, 9 (12) 2208-2214
- Monajjemi, M.; Honarparvar, B.; Nasseri, S. M. .; Khaleghian M. Journal of Structural Chemistry. 2009, 50(1), 67-77
- Monajjemi, M.; Aghaie, H.; Naderi, F. Biochemistry (Moscow).2007,72 (6), 652-657
- Ardalan, T.; Ardalan, P.; Monajjemi, M. Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22: 687–708
- Mollaamin, F.; Monajjemi, M.; Mehrzad, J. Fullerenes, Nanotubes, and Carbon Nanostructures. 2014, 22: 738–751
- Monajjemi, M.; Najafpour, J.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures. 2013, 21(3), 213–232
- Monajjemi, M.; Karachi, N.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22: 643–662
- Yahyaei, H.; Monajjemi, M. Fullerenes, Nanotubes, and Carbon Nanostructures.2014, 22(4), 346–361
- Monajjemi, M. Falahati, M.; Mollaamin, F.; Ionics, 2013, 19, 155–164
- Monajjemi, M.; Mollaamin, F. Journal of Cluster Science, 2012, 23(2), 259-272
- Tahan, A.; Monajjemi, M. Acta Biotheor,2011, 59, 291–312
- Lee, V.S.; Nimmanpipug, P.; Mollaamin, F.; Kungwan, N.; Thanasanvorakun, S.; Monajjemi, M. Russian Journal of Physical Chemistry A, 2009, 83(13), 2288–2296
- Monajjemi, M.; Heshmat, M.; Haeri, HH, Biochemistry (Moscow), 2006, 71 (1), S113-S122
- Monajjemi, M.; Yamola, H.; Mollaamin, F.Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22, 595–603
- Mollaamin, F.; Layali, I.; Ilkhani A. R.; Monajjemi, M. African Journal of Microbiology Research .2010, 4(24) 2795-2803
- Mollaamin, F.; Shahani poor, p K. .; Nejadsattari, T. ; Monajjemi, M. African Journal of Microbiology Research. 2010, 4(20) 2098-210
- Monajjemi, M.; Ahmadianarog, M. Journal of Computational and Theoretical Nanoscience. 2014, 11(6), 1465-1471
- Monajjemi, M.; JafariAzan, M.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures.2013, 21(6), 503–515
- Mollaamin, F.; Monajjemi, M.Physics and Chemistry of Liquids .2012,50(5), 596–604
- Monajjemi, M.; Khosravi, M.; Honarparvar, B.; Mollaamin, F.; International Journal of Quantum Chemistry, 2011, 111, 2771–2777
- Khaleghian, M.; Zahmatkesh, M.; Mollaamin, F.; Monajjemi, M. Fullerenes, Nanotubes, and Carbon Nanostructures, 2011, 19(4): 251–261
- Monajjemi, M.; Baheri, H.; Mollaamin, F. Journal of Structural Chemistry.201152(1), 54-59
- Mahdavian, L.; Monajjemi, M.; Mangkorntong, N. Fullerenes, Nanotubes and Carbon Nanostructures, 2009, 17 (5), 484-495
- Monajjemi, M., Mahdavian, L., Mollaamin, F. Bull. Chem. Soc. Ethiop. 2008, 22(2), 277-286
- Monajjemi, M.; Afsharnezhad, S, Jaafari, M.R..; Mirdamadi, S..; Mollaamin, F..; Monajemi, H. Chemistry .2008, 17 (1), 55-69
- Monajjemi, M.; Mollaamin,F.; Gholami, M. R.; Yoozbashizadeh,H.; Sadrnezhaad, S.K.; Passdar, H.;Main Group Metal Chemistry,2003, 26 (6), 349-361
- Monajjemi, M.; Azad ,MT.; Haeri, HH.; Zare, K.; Hamedani, Sh.; Journal Of Chemical Research-S.2003,8: 454-456
- Monajjemi, M.; Najafpour, J. Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22(6): 575–594
- Monajjemi, M.; Noei, M.; Mollaamin, F.Nucleosides, Nucleotides and Nucleic Acids. 2010 29(9):676–683
- Ghiasi, R.; Monajjemi, M.Journal of Sulfur Chemistry .2007, 28(5), 505-511
- Monajjemi, M.; Ghiasi, R.; Abedi, A. Russian Journal of Inorganic Chemistry.2005, 50(3), 382-388
- Monajjemi, M. .; Naderi, F.; Mollaamin, F.; Khaleghian, M. J. Mex. Chem. Soc. 2012, 56(2), 207-211
- Monajjemi, M.; Farahani, N.; Mollaamin, F. Physics and Chemistry of Liquids, 2012, 50(2) 161–172
- Monajjemi, M.; SeyedHosseini, M. Journal of Computational and Theoretical Nanoscience .2013, 10(10), 2473-2477
- Monajjemi , M.; Honaparvar , B.; KhaliliHadad ,B.; Ilkhani ,AR.; Mollaamin, F. African Journal of Pharmacy and Pharmacology .2010, 4(8), 521 -529
- Monajjemi, M. TheorChemAcc, 2015, 134:77 DOI 10.1007/s00214-015-1668-9
- Monajjemi, M. Journal of Molecular Modeling, 2014, 20, 2507
- Monajjemi, M.; Honarparvar, B.; Monajemi, H.; Journal of the Mexican Chemical Society, 2006, 50 (4), 143-148
- Monajjemi, M.; Khaleghian, M.; Mollaamin, F. Molecular Simulation. 2010, 36(11), 865–
- Ilkhani, Ali R.; Monajjemi, M. Computational and Theoretical Chemistry.20151074, 19–25
- Monajjemi, M. Biophysical Chemistry.2015207,114 –127
- Monajjemi, M., Moniri, E., Panahi, H.A ,Journal of Chemical and Engineering Data.2001,1249-1254.
- Mollaamin, F.;Najafpour, J.;Ghadami, S.;Ilkhani, A. R.;Akrami, M. S.;Monajjemi, M. Journal of Computational and Theoretical Nanoscience.11 (5), 1290-1298
- Monajjemi, M.; Ghiasi, R.; Ketabi, S.; Passdar, H.; Mollaamin, F.Journal of Chemical Research .2004, 1, 11.
- Monajjemi, M.; Heshmat, M.; Haeri, H.H. Biochemistry (Moscow).2006, 71, 113-122
- Monajjemi, M.; Heshmat, M.; Aghaei, H.;Ahmadi, R.; Zare, K. Bulletin of the Chemical Society of Ethiopia. 2007, 21, 111–116
- Monajjemi, M., Kharghanian, L., Khaleghian, M., Chegini, H.Fullerenes Nanotubes and Carbon Nanostructures.2014, 22,(8), 0.1080/1536383X.2012.717563
- Sarasia, E.M.; Afsharnezhad, S.; Honarparvar, B.; Mollaamin, F.; Monajjemi, M. Physics and Chemistry of Liquids.2011, 49 (5), 561-571
- Amiri, A.; Babaeie, F.; Monajjemi, M.Physics and Chemistry of Liquids. 2008, 46(4), 379-389
- Monajjemi, M.; Heshmat, M.; Haeri, H.H.; Kaveh, F. Russian Journal of Physical Chemistry A,2006, 80(7), 1061-1068
- Monajjemi, M.; Moniri, E.; Azizi, Z.; Ahmad Panahi, H. Russian Journal of Inorganic Chemistry. 2005, 50(1), 40-44
- Jalilian,H.; Monajjemi, M. Japanese Journal of Applied Physics. 2015,54 (8), 08510
- Mollaamin, F.;Monajjemi, M. Journal of Computational and Theoretical Nanoscience. 2015, 12(6), 1030-1039
- Felegari, Z.; Monajjemi, M. Journal of Theoretical and Computational Chemistry. 2015,14(3), 1550021
- Shojaee, S., Monajjemi, M.Journal of Computational and Theoretical Nanoscience. 2015,12(3), 449-458
- Esmkhani, R.;Monajjemi, M. Journal of Computational and Theoretical Nanoscience. 2015. 12(4), 652-659
- Monajjemi, M., Seyedhosseini, M., Mousavi, M., Jamali, Z,Journal of Computational and Theoretical Nanoscience. 2015, 23(3), 239-244
- Ghiasi, R.; Monajjemi, M.; Mokarram, E.E.; Makkipour, P. Journal of Structural Chemistry. 2008, 4(4), 600-605
- Mahdavian, L.; Monajjemi, M. Microelectronics Journal. 2010,41(2-3), 142-149
- Monajjemi, M.; Baie, M.T.; Mollaamin, F. Russian Chemical Bulletin.2010, 59(5), 886-889
- Bakhshi, K.; Mollaamin, F.; Monajjemi, M. Journal of Computational and Theoretical Nanoscience. 2011, 8(4),763-768
- Darouie, M.; Afshar, S.; Zare, K., Monajjemi, M. journal of Experimental Nanoscience.2013, 8(4),451-461
- Amiri, A.; Monajjemi, M.; Zare, K.; Ketabi, S.Physics and Chemistry of Liquids. 2006, 44(4), 449-456.
- Zonouzi, R.;Khajeh, K.; Monajjemi, M.; Ghaemi, N. Journal of Microbiology and Biotechnology. 2013,23(1), 7-14
- Ali R. Ilkhani.; MajidMonajjemi, Computational and Theoretical Chemistry.2015, 10(74) 19–25
- Tahan, A.; Mollaamin, F.; Monajjemi, M. Russian Journal of Physical Chemistry A, 2009, 83 (4), 587-597
- KhaliliHadad, B.; Mollaamin, F.; Monajjemi, M, Russian Chemical Bulletin,2011, 60(2):233-236
- Mollaamin, F.; Monajjemi, M.; Salemi, S.; Baei, M.T. Fullerenes Nanotubes and Carbon Nanostructures, 2011, 19(3), 182-196
- Mollaamin, F.; Shahani Pour.; K., Shahani Pour, K.; ilkhani, A.R.; Sheckari, Z., Monajjemi, M Russian Chemical Bulletin , 2012 , 61(12), 2193-2198
- Shoaei, S.M.; Aghaei, H.; Monajjemi, M.; Aghaei, M. Phosphorus, Sulfur and Silicon and the Related Elements. 2014, 189(5) 652-660
- Mehrzad, J., Monajjemi, M., Hashemi, M ,Biochemistry (Moscow).2014 , 79 (1), 31-36
- Moghaddam, N.A., Zadeh, M.S., Monajjemi, M. Journal of Computational and Theoretical Nanoscience ,2015 , 12(3), doi:10.1166/jctn.2015.3736
- Joohari, S.; Monajjemi, M, Songklanakarin Journal of Science and Technology, 2015, 37(3):327
- Rajaian, E., Monajjemi, M., Gholami, M.R, Journal of Chemical Research – Part S, 2002, 6(1), 279-281
- Ghassemzadeh, L., Monajjemi, M., Zare, K,Journal of Chemical Research – Part S, 2003,4, 195-199
- Mehdizadeh Barforushi,M.; Safari,S.; Monajjemi,M.;J. Comput. Theor.Nanosci.2015, 12, 3058-3065.
- Mollaamin,F.; Ilkhani,A.; Sakhaei,N.; Bonsakhteh,B.; Faridchehr,A.; Tohidi,S.; Monajjemi,M.; J. Comput. Theor.Nanosci.2015, 12, 3148-3154.
- Rahmati,H.; Monajjemi,M.; J. Comput. Theor. Nanosci.2015, 12, 3473-3481.
- TarlaniBashiz,R.; Monajjemi,M.; J. Comput. Theor. Nanosci.2015,12, 3808-3816.
- MehrabiNejad,A.; Monajjemi,M.; J. Comput. Theor.Nanosci.2015, 12, 3902-3910.
- Monajjemi,M *.; Bagheri,S.; Moosavi,M.S..; Moradiyeh,N.; Zakeri,M.; Attarikhasraghi,N.; Saghayimarouf,N.; Niyatzadeh,G.; Shekarkhand,M.; Mohammad S. Khalilimofrad, Ahmadin,H.; Ahadi,M.; Molecules 2015, 20, 21636–21657;
- Shabanzadeh,E.; Monajjemi,M.; J. Comput. Theor. Nanosci.2015, 12, 4076-4086.
- Elsagh,A.: Jalilian,H.; Kianpour,E.; Sadat Ghazi Mokri,H.; Rajabzadeh,M.; Moosavi,M.S.; GhaemiAmiri,F.; Monajjemi,M.; J. Comput. Theor.Nanosci.2015, 12, 4211-4218.
- Faridchehr,A.; Rustaiyan, A.; Monajjemi,M.; J. Comput. Theor. Nanosci.2015, 12, 4301-4314.
- Tohidi,S.; Monajjemi,M.; Rustaiyan,A.; J. Comput. Theor.Nanosci.2015, 12, 4345-4351.
- Ali AkbariZadeh,M.; Lari,H.; Kharghanian,L.; Balali,E.; Khadivi,R.; Yahyaei,H.; Mollaamin,F.; Monajjemi,M.; J. Comput. Theor.Nanosci.2015, 12, 4358-4367.
- Dezfooli,S.; Lari,H.; Balali,E.; Khadivi,R.; Farzi,F.; Moradiyeh,N.; Monajjemi,M.; J. Comput. Theor.Nanosci.2015, 12, 4478-4488.
- Jalilian,H.: Sayadian,M.; Elsagh,A.; Farzi,F.; Moradiyeh,N.; SamieiSoofi,N.; Khosravi,S.; Mohammadian,N.T.; Monajjemi,M.;J. Comput. Theor. Nanosci.2015, 12, 4785-4793.
- Farzi,F.; Bagheri,S.; Rajabzadeh,M.; Sayadian,M.; Jalilian,H.; Moradiyeh,N.; Monajjemi,M.; J. Comput. Theor.Nanosci.2015, 12, 4862-4872.
- Monajjemi,M.; Nayyer T. MohammadianJ. Comput. Theor.Nanosci.2015,12, 4895-4914.
- Monajjemi, M., Chahkandi, B. Journal of Molecular Structure: THEOCHEM, 2005, 714 (1), 28, 43-60.
- Joohari*,S.; Monajjemi, M.; Bulgarian Chemical Communications,2015, 47(2) 631 – 646.
- Moradiyeh,N.; Zakeri, M.; Attarikhasraghi,N.; Ahadi,M.; Saghayimarouf ,N.; Niyatzadeh , G.; Mahmoodi ,Z.; Shekarkhand,M.; Ahmadin, H.; Monajjemi,M.;J. Comput. Theor.Nanosci.2015, 12, 5395–5401.
- Zawari, M.; Haghighizadeh, M.; Derakhshandeh,M.; Barmaki ,Z.; Farhami,N.; Monajjemi,M.; J. Comput. Theor.Nanosci.2015, 12,5472–5478.
- Sadatchoobeh,S.; Monajjemi,M.; J. Comput. Theor.Nanosci.2015, 12,5789–5795.
- Shadmani ,N.; MehdizadehBarforushi, M.; Shakibayifar, J.; Elsagh,A.; Zare ,K.; Abbasi,Z.; Khalili1 ,M.S.; Ahmadin ,H.; Rajabzadeh,M.; Sayadian,M.; Monajjemi,M.; J. Comput. Theor.Nanosci.2016, 13, 208–219.
- Shadmani,N.; Monajjemi,M.; Zare,K.; J. Comput. Theor.Nanosci.2016, 13, 378–387.
- Grimme, S. AngewChemInt Ed, 2006, (45), 4460–4464, DOI: 10.1002/anie.200600448.
- Schreiner, P.R.;Fokin, A. A.; Pascal, R. A Jr.; de Meijere, A.Org. Lett, 2006, (8): 3635–3638.
- Zhao, Y.;Truhlar, D.G. Org. Lett, 2006, (8):5753–5755.
- Kohn, W.; Sham, LJ. Phys. Rev, 1965,(140)A: 1133-1138.
- Perdew, J .P. Burke, K.; Phys. Rev. Lett.1996, (77): 3865-3868.
- Besler, B.H.; Merz, K.M.; Kollman, P.A. J. comp. Chem, 1990, (11): 431-439,
- Chirlian, L.E.;Francl, M.M.J.comp.chem,1987, (8): 894-905,
- Brneman, G.M, Wiberg, K.B. J. Comp Chem, 1990, (11): 361
- Martin,F.;Zipse, H. J Comp Chem. 2005, (26): 97 – 105.
- Balderchi, A.;Baroni, S.;Resta, R. Phys. Rev. Lett, 1998, (61): 173.

This work is licensed under a Creative Commons Attribution 4.0 International License.









