In Vitro Antibacterial, Antifungal and Phytochemical Analysis of Methanolic Extract of Fruit Cassia Fistula
Mohanad Jawad Kadhim1, Ghaidaa Jihadi Mohammed2 and Imad Hadi Hameed3*
1Department of Genetic Engineering, Al-Qasim Green University, Iraq
2College of Science, Al-Qadisyia University, Iraq
3College of Nursing, Babylon University, Iraq
Corresponding Author E-mail: imad_dna@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/320307
Article Received on : April 02, 2016
Article Accepted on : May 05, 2016
Article Published : 24 May 2016
The identification of phytochemical compounds is based on the peak area, retention time molecular weight, molecular formula, MS Fragment- ions and Pharmacological actions. GC-MS analysis of Cassia fistula revealed the presence of the Oxacyclododecan-2-one, Imidazole ,2-amino-5-[(2-carboxy)vinyl], D-Glucose , 6-O-α-D-galactopyranosyl, 2-Nonanone, Eicosanoic acid , phenylmethyl ester, Phenol , 4-(2-propenyl), Eugenol, Caryophyllene, ß-copaene, Azulene,1,2,3,3a,4,5,6,7-octahydro-1,4-dimethyl-7-(1-methylethenyl), α-acorenol, Spiro[5.5]undec-8-en-1-one, Isoaromadendrene epoxide, Tetraacetyl-d-xylonic nitrile , Benzyl Benzoate, N-Isobutyl-(2E,4Z,8Z,10E)-dodecatetraenamide, Phenethylamine , 3-benzyloxy-2-fluoro-ß-hydroxy, 4a-Hydroxy-4-nitroperhydronaphthalen-1-one, Dasycarpidan -1-methanol, acetate (ester), Propanoic acid , 2-(3-acetoxy-4,4,14-trimethylandrost-8-en-17-yl), Carda-4,20(22)-dienolide,3-[(6-deoxy-3-O-methyl-α-L-mannopyranosyl, Cis-13-Eicosenoic acid , 16-Nitrobicyclo[10.4.0]hexadecane-1-ol-13-one, Strychane ,1-acetyl-20α-hydroxy-16-methylene, 2,4,6-Decatohols, Ethers, Carboxlic arienoic acid , 1a,2,5,5a,6,9,10,10a-octahydro-5,5a-dihydro, Vitamin E and Glycine ,N-[(3α,5ß,12α)-3,12-dihydroxy-24-oxocholan-24-yl]. The FTIR analysis of Cassia fistula leaves proved the presence of Alkenes, Aliphatic fluoro compounds, Alccids, Esters, Nitro Compounds, Alkanes, Alcohols and Phenol. Cassia fistula was highly active against Aspergillus terreus (6.99±0.29). Methanolic extract of bioactive compounds of Cassia fistula was assayed for in vitro antibacterial activity against eleven pathogenic bacteria by using the diffusion method in agar. The zone of inhibition were compared with different standard antibiotics. The diameters of inhibition zones ranged from 1.00±0.05 to 6.02±0.23 mm for all treatments.
KEYWORDS:GC/MS; Bioactive compounds; FT-IR; Cassia fistula
Download this article as:| Copy the following to cite this article: Kadhim M. J, Mohammed G. J, Hameed I. H. In Vitro Antibacterial, Antifungal and Phytochemical Analysis of Methanolic Extract of Fruit Cassia Fistula. Orient J Chem 2016;32(3). |
| Copy the following to cite this URL: Kadhim M. J, Mohammed G. J, Hameed I. H. In Vitro Antibacterial, Antifungal and Phytochemical Analysis of Methanolic Extract of Fruit Cassia Fistula. Orient J Chem 2016;32(3). Available from: http://www.orientjchem.org/?p=16287 |
Introduction
Cassia fistula plant have naturally occurring bioactive compounds and are mostly secondary metabolites which are now a days being used as medicines, dietary supplements and other useful commercial products1. Also, has been reported to contain anthraquinone the principal laxative constituent of many plants used as purgative2. Plants have been an important source of medicine with qualities for thousands of years3,4. Mainly on traditional remedies such as herbs for their history, they have been used as popular folk medicines5,6. Since thousands of years back, plants are used as a major source for medicine as they found to possess a reservoir of bioactive compound7-9. Cassia fistula contains alkaloids, tannins, flavonoids,terpenes, sugars, and glucosides. Tannins are naturally occurring and water soluble phenolic compounds, which precipitate proteins from aqueous media10. Cassia fistula shows the presence of glycoside a natural product, which is used to enhance the cardiac contractile force in patient with congestive heart failure11 glycoside also plays major role in the cancer therapy12. They also play major role in controlling topical disease as eczema etc. Many metabolites have found to possess interesting biological activities such as bactericidal, fungicidal, hepatoprotective and muscle relaxant13. A fruit is cylindrical pod and seeds many in black, sweet pulp separated by transverse partitions. The long pods which are green, when unripe, turn black on ripening after flowers shed14. Pulp is dark brown in colour, sticky, sweet and mucilaginous, odour characteristic, and somewhat disagreeable15. Each compartment contains one seed which is flat, oval, reddish brown with a well-marked raphe. The seed contains a whitish endosperm in which the yellowish embryo is embedded16. Fruits are used in the treatment of diabetes, antipyretic, abortifacient, demulcent, lessens inflammation and heat of the body; useful in chest complaints, throat troubles, liver complaints, diseases of eye and gripping17. The aim of this research was study the phytochemical composition of Cassia fistula and to evaluate the isolates for possible in vitro antifungal and antibacterial activities.
Materials and Methods
Solvent extraction
The shade dried powdered of Cassia fistula was extracted with methanol. The extract was filtered with Whatman’s filter paper. Filtrate was concentrated under reduced pressure and preserved at 5Cº in dark air tight bottles18,19.
Sample preparation for GC-MS analysis
50 μl of sample (Cassia fistula) was dissolved in 2ml of methanol and kept in ultrasonic bath for 25 min and centrifuged for 10 min. at 6000 rpm and supernatant was injected in GC-MS for analysis20,21.
Gas chromatography-mass spectrometry (GC-MS) analysis
GC-MS of methanol extract was performed using Agilent 7890A. The run time was 30 minutes. ionization of sample components were performed on EI mode (70 eV). The carrier gas was helium at 1.0ml/min flow rate. 0.5 ml of sample was injected in split mode of 20:1.The mass spectrum scan range was set at 29.0 to 500(m/z) 22.
Identification of compounds
Interpretation of mass spectrum of GC-MS was done using the database of National Institute Standard and Technology (NIST). The mass spectrum of phytochemicals was compared with the spectrum of known compounds stored in the NIST library23.
Determination of antibacterial activity of crude bioactive compounds of Cassia fistula
The anti-bacterial activity was evaluated using Mueller-Hinton agar. The bacterial plates were incubated at 37 °C for 24 h. After incubation, the diameter of the inhibition zone was measured to evaluate the antimicrobial activity. Each test was performed twice and the average of the results was calculated. The extraction solvents were used as negative control24,25. The test pathogens were swabbed in Muller Hinton agar plates. 60μl of plant extract was loaded on the bored wells. The wells were bored in 0.5 cm in diameter. The plates were incubated at 37C° for 24 h and examined. After the incubation the diameter of inhibition zones around the discs was measured26.
Determination of antifungal activity
Five-millimeter diameter wells were cut from the agar using a sterile cork-borer, and 50 μl of the samples solutions Cassia fistula was delivered into the wells. Antimicrobial activity was evaluated by measuring the zone of inhibition against the test microorganisms. Methanol was used as solvent control. Amphotericin B and fluconazole were used as reference antifungal agent. The tests were carried out in triplicate. The antifungal activity was evaluated by measuring the inhibition-zone diameter observed after 48 h of incubation27,28.
Statistical analysis
Results of the study were based on analysis of variance (ANOVA) and differences were considered significant at p < 0.05.
Results and Discussion
Identification of phytochemical compounds
Plants have been a common source of medicinal property, either in the form of pure active compound or as traditional preparations and it are reasonable to use local plants29. Identifying on the plant phytochemistry provides a fundamental use of plants as storage of chemical agents in the field of medicine13. The phytochemical analyses of plant from Cassia fistula was studied extensively. Identifying the importance of secondary metabolites in the field of medicine, the presence of tannins, phlobatanins, saponins, flavonoids, terpenoids, glycosides and steroids was detected. Folklore medicine are widely used in our ancient period13. Gas chromatography and mass spectroscopy analysis of compounds was carried out in methanolic leaves extract of Cassia fistula, shown in Table 1. The GC-MS chromatogram of the 27 peaks of the compounds detected was shown in Figure 1. Chromatogram GC-MS analysis of the methanol extract of Cassia fistula showed the presence of twenty seven major peaks and the components corresponding to the peaks were determined as follows. The first set up peak were determined to be 1,7-Dioxaspiro[5,5]undec-2-ene Figure 2. The second peak indicated to be 2,4-Dihydroxy-2,5-dimethyl-39(2H)-furan-3-one Figure 3. The next peaks considered to be α-D-Glucopyranoside , O-α-D-glucopyranosyl-(1.fwdarw.3)-ß-D-fruc, d-Mannose, 5,7-Dodecadiyn -1,12-diol, 3-Trifluoroacetoxypentadecane, 3-Trifluoroacetoxypentadecane, Pterin-6-carboxylic acid, Imidazole-4-carboxylic acid ,2-fluoro-1-methoxymethyl-,ethyl ester, D-Carvone, Pyrrolizin-1,7-dione-6-carboxylic acid , methyl (ester), D-Glucose ,6-O-α-D-galactopyranosyl, Estragole, Phenol,2-methyl-5-(1-methylethyl), 3-Allyl-6-methoxyphenol, Ppropiolic acid , 3-(1-hydroxy-2-isopropyl-5-methylcyclohexyl), 7-epi-trans-sesquisabinene hydrate, Tetraacetyl-d-xylonic nitrile, y-Sitosterol, Ergosta-5,22-dien-3-ol, acetate , (3ß,22E), Curan-17-oic acid ,2,16-didehydro-20-hydroxy-19-oxo,methyl ester, 9,10-Secocholesta -5,7,10(19)-triene-1,3-diol,25-[(trimethylsilyl)oxy], Cis-Vaccenic acid, L-Ascorbic acid , 6-octadecanoate, L-Ascorbic acid , 6-octadecanoate, Deoxyspergualin, Tributyl acetylcitrate, 10,13-Dioxatricyclo[7.3.1.0(4,9)]tridecan-5-ol-2-carboxylic acid, 18,19-Secoyohimban-19-oic acid , 16,17,20,21-tetradehydro-16, 9-Octadecenamide ,(Z), Olean-12-ene-3,15,16,21,22,28,-hexol,(3ß,15α,16α,21ß,22α), (22S)-21-Acetoxy-6α,11ß-dihydroxy-16α,17αpropylmethylenedioxy, Ethyl iso-allocholate, Olean-12-ene-3,15,16,21,22,28-hexol,(3ß,15α,16α,21ß,22α) and Olean -13(18)-ene (Figure 3-28). The FTIR analysis of Cassia fistula leaves proved the presence of alkenes, aliphatic fluoro compounds, alcohols, ethers, carboxlic acids, esters, nitro compounds, hydrogen bonded alcohols and phenols which shows major peaks at 675.09, 904.61, 1014.56, 1072.42, 1242.16, 1317.38, 1361.74, 2918.30 and 3277.06 (Table 2; Figure 29). Cassia fistula Linn. (Cassia) family Caesalpiniaceae commonly known as Amulthus has been extensively used in Ayurvedic system of medicine for various ailments30. Cassia fistula is widely used in traditional medicinal system of India has been reported to possess hepatoprotective, anti-inflammatory, antitussive, antifungal and used also check wounds healing and antibacterial15. Fruits are used as catharatic and in snake bite. Juice of leaves is used in skin diseases31-33. Flowers and pods are used as purgative, febrifugal, biliousness and astringent. The ethanolic 50% extract of pods show antifertility activity in female albino rats. The heated pods are applied to swellings on the neck due to cold. The fruits are reported to be used for asthma. Padma, (2006) 17 tested the methanolic leaf extract of Cassia fistula for activity against Candida albicans showed highest activity i.e., upto 21 mm which was comparable with the standard antifungal antibiotic, clotrimazole.
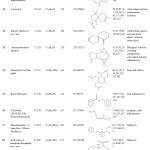 |
Table 1: Major phytochemical compounds identified in methanolic extract of Cassia fistula.
|
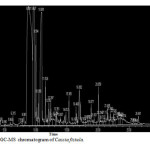 |
Figure 1: GC-MS chromatogram of Cassia fistula
|
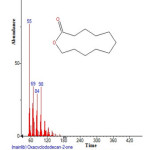 |
Figure 2: Structure of Oxacyclododecan-2-one with RT: 3.367 present in Cassia fistula.Click here to View figure |
![Figure 3. Structure of Imidazole ,2-amino-5-[(2-carboxy)vinyl] with RT: 3.613 present in Cassia fistula.](http://www.orientjchem.org/wp-content/uploads/2016/05/Vol32No3_-vitr_MOH-_FIG-3-150x150.jpg) |
Figure 3: Structure of Imidazole ,2-amino-5-[(2-carboxy)vinyl] with RT: 3.613 present in Cassia fistula. Click here to View figure |
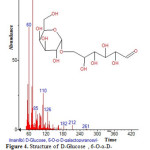 |
Figure 4: Structure of D-Glucose , 6-O-α-D-galactopyranosyl with RT: 3.751 present in Cassia fistula. Click here to View figure |
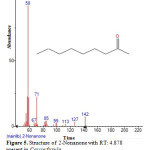 |
Figure 5: Structure of 2-Nonanone with RT: 4.878 present in Cassia fistula. Click here to View figure |
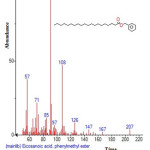 |
Figure 6: Structure of Eicosanoic acid , phenylmethyl ester with RT: 5.833 present in Cassia fistula. Click here to View figure |
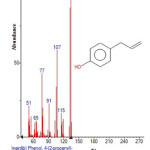 |
Figure 7: Structure of Phenol , 4-(2-propenyl) with RT: 7.344 present in Cassia fistula Click here to View figure |
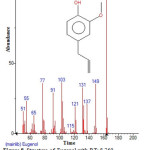 |
Figure 8: Structure of Eugenol with RT: 8.769 present in Cassia fistula. Click here to View figure |
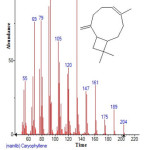 |
Figure 9: Structure of Caryophyllene with RT: 9.644 present in Cassia fistula. Click here to View figure |
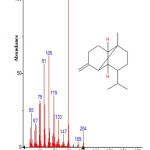 |
Figure 10: Structure of ß-copaene with RT: 10.606 present in Cassia fistula. Click here to View figure |
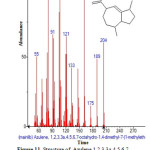 |
Figure 11: Structure of Azulene,1,2,3,3a,4,5,6,7-octahydro-1,4-dimethyl-7-(1-methylethenyl) with RT: 10.411 present in Cassia fistula. Click here to View figure |
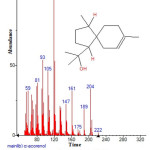 |
Figure 12: Structure of α-acorenol with RT: 10.331 present in Cassia fistula. Click here to View figure |
![Figure 13. Structure of Spiro[5.5]undec-8-en-1-one with RT: 11.183 present in Cassia fistula.](http://www.orientjchem.org/wp-content/uploads/2016/05/Vol32No3_-vitr_MOH-_FIG-13-150x150.jpg) |
Figure 13: Structure of Spiro[5.5]undec-8-en-1-one with RT: 11.183 present in Cassia fistula. Click here to View figure |
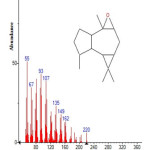 |
Figure 14: Structure of Isoaromadendrene epoxide with RT: 12.253 present in Cassia fistula. Click here to View figure |
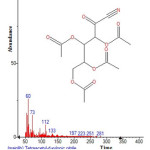 |
Figure 15: Structure of Tetraacetyl-d-xylonic nitrile with RT: 12.797 present in Cassia fistula. Click here to View figure |
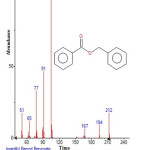 |
Figure 16: Structure of Benzyl Benzoate with RT: 13.346 present in Cassia fistula. Click here to View figure |
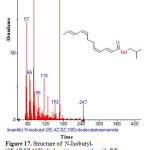 |
Figure 17: Structure of N-Isobutyl-(2E,4Z,8Z,10E)-dodecatetraenamide with RT: 14.228 present in Cassia fistula. |
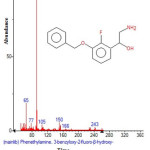 |
Figure 18: Structure of Phenethylamine , 3-benzyloxy-2-fluoro-ß-hydroxy with RT: 14.416 present in Cassia fistula. Click here to View figure |
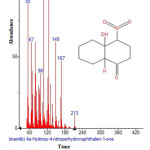 |
Figure 19: Structure of 4a-Hydroxy-4-nitroperhydronaphthalen-1-one with RT: 15.468 present in Cassia fistula. Click here to View figure |
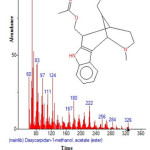 |
Figure 20: Structure of Dasycarpidan -1-methanol, acetate (ester) with RT: 15.870 present in Cassia fistula. Click here to View figure |
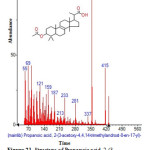 |
Figure 21: Structure of Propanoic acid, 2-(3-acetoxy-4,4,14-trimethylandrost-8-en-17-yl) with RT: 16.745 present in Cassia fistula. Click here to View figure |
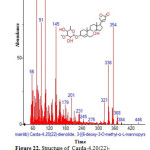 |
Figure 22: Structure of Carda-4,20(22)-dienolide,3-[(6-deoxy-3-O-methyl-α-L-mannopyranosyl with RT: 18.473 present in Cassia fistula. Click here to View figure |
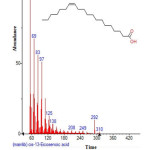 |
Figure 23: Structure of Cis-13-Eicosenoic acid with RT: 18.611 present in Cassia fistula. Click here to View figure |
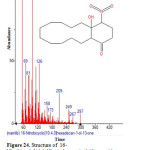 |
Figure 24: Structure of 16-Nitrobicyclo[10.4.0]hexadecane-1-ol-13-one with RT: 19.080 present in Cassia fistula. Click here to View figure |
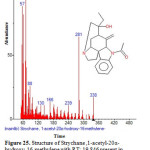 |
Figure 25: Structure of Strychane ,1-acetyl-20α-hydroxy-16-methylene with RT: 19.846 present in Cassia fistula. Click here to View figure |
 |
Figure 26: Structure of 2,4,6-Decatrienoic acid , 1a,2,5,5a,6,9,10,10a-octahydro-5,5a-dihydro with RT: 21.929 present in Cassia fistula. Click here to View figure |
 |
Figure 27: Structure of Vitamin E with RT: 26.484 present in Cassia fistula. Click here to View figure |
![Figure 28. Structure of Glycine ,N-[(3α,5ß,12α)-3,12-dihydroxy-24-oxocholan-24-yl] with RT: 28.893 present in Cassia fistula.](http://www.orientjchem.org/wp-content/uploads/2016/05/Vol32No3_-vitr_MOH-_FIG-281-150x150.jpg) |
Figure 28: Structure of Glycine ,N-[(3α,5ß,12α)-3,12-dihydroxy-24-oxocholan-24-yl] with RT: 28.893 present in Cassia fistula. Click here to View figure |
 |
Table 2: FT-IR peak values of Cassia fistula |
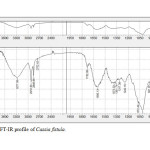 |
Figure 29: FT-IR profile of Cassia fistula. Click here to View table |
Evaluation of antimicrobial activity
In the current study, the anti-microbial activity of the methanolic extract was evaluated by determining the zone of inhibition against five bacteria and fourteen fungi and yeast. Clinical pathogens were selected for antibacterial activity namely, (Bacillus subtilis, Pseudomonas eurogenosa, Streptococcus faecalis, Salmonella typhi and Staphylococcus aureus. Maximum zone formation was against Streptococcus faecalis. Methanolic extraction of plant showed notable antifungal activities against Aspergillus niger, Aspergillus terreus, Aspergillus flavus, Aspergillus fumigatus, Candida albicans, Saccharomyces cerevisiae, Fusarium sp., Microsporum canis, Streptococcus faecalis, Mucor sp., Penicillium expansum, Trichoderma viride, Trichoderma horzianum and Trichophyton mentagrophytes. Cassia fistula was very highly active against Aspergillus terreus (7.09±0.32). Results of antimicrobial activity are presented in Table 3. In comparison to the antibiotics used in this study, the plants extracts were far more active against the test bacterial strains.
Table 3: Antimicrobial activity of Cassia fistula.
| Microbe | Plant |
Cefotoxime |
Streptomycin |
Amphotericin B |
Fluconazol |
||||
| Streptococcus pneumonia |
4.19±0.15 |
1.00±0.07 |
2.00±0.11 |
– |
– |
||||
| Pseudomonas eurogenosa |
5.73±0.20 |
1.30±0.09 |
1.07±0.06 |
– |
– |
||||
| Staphylococcus epidermidis |
3.00±0.10 |
1.31±0.09 |
1.56±0.09 |
– |
– |
||||
| Salmonella typhi |
4.15±0.14 |
1.01±0.08 |
1.22±0.08 |
– |
– |
||||
| Bacillus subtilis |
4.00±0.12 |
1.49±0.10 |
1.00±0.04 |
– |
– |
||||
| Escherichia coli |
4.83±0.19 |
2.03±0.11 |
2.08±0.10 |
– |
– |
||||
| Proteus mirabilis |
6.02±0.23 |
1.90±0.09 |
1.00±0.05 |
– |
– |
||||
| Streptococcus pyogenes |
3.04±0.11 |
1.16±0.09 |
1.82±0.05 |
– |
– |
||||
| Staphylococcus aureus |
5.20±0.20 |
2.00±0.10 |
2.11±0.12 |
– |
– |
||||
| Streptococcus faecalis |
4.65±0.20 |
1.58±0.08 |
1.00±0.05 |
– |
– |
||||
| Klebsiella pneumonia |
5.23±0.23 |
1.27±0.07 |
1.95±0.09 |
– |
– |
||||
| Fungi/ Yeast | |||||||||
|
Aspergillus niger |
5.89±0.22 |
– |
– |
1.94±0.08 |
2.11±0.19 |
||||
|
Aspergillus terreus |
6.99±0.29 |
– |
– |
4.00±0.16 |
1.95±0.18 |
||||
|
Aspergillus flavus |
5.96±0.28 |
– |
– |
2.00±0.20 |
3.00±0.22 |
||||
|
Aspergillus fumigatus |
6.00±0.29 |
– |
– |
2.06±0.09 |
2.85±0.17 |
||||
| Candida albicans |
5.25±0.20 |
– |
– |
2.00±0.19 |
2.00.±0.11 |
||||
| Saccharomyces cerevisiae |
3.92±0.18 |
– |
– |
1.89±0.09 |
2.90±0.19 |
||||
| Fusarium sp. |
5.01±0.24 |
– |
– |
2.99±0.10 |
3.00±0.21 |
||||
| Microsporum canis |
4.00±0.19 |
– |
– |
3.31±0.19 |
1.97±0.10 |
||||
| Streptococcus faecalis |
3.80±0.27 |
– |
– |
3.73±0.14 |
2.60±0.20 |
||||
| Mucor sp. |
4.00±0.19 |
– |
– |
2.00±0.17 |
1.99±0.18 |
||||
| Penicillium expansum |
4.12±0.19 |
– |
– |
2.61±0.19 |
2.60±0.17 |
||||
| Trichoderma viride |
5.08±0.23 |
1.99±0.15 |
2.30±0.18 |
||||||
| Trichoderma horzianum |
3.95±0.17 |
0.94±0.01 |
2.99±0.19 |
||||||
| Trichophyton mentagrophytes |
4.00±0.19 |
2.71±0.14 |
1.00±0.14 |
||||||
ª The values (average of triplicate) are diameter of zone of inhibition at 100 mg/mL crude extract, 30 μg/mL of antibiotics (Streptomycin; Rifambin; Kanamycin; Cefotoxime and chloramphenicol).
Conclusion
Medicinal property of plant extract is due to presence of secondary metabolites. Twenty seven phytoconstituents were identified from methanol extract of Cassia fistula by gas chromatogram and mass spectrometry (GC-MS) analysis. This plant derived bioactive compounds used as source of antibiotic properties and pharmaceutical industries used for drug formulation. This plant crude extract showed the phytochemical constituent has great potential for food resource and malnutrition of human health.
Acknowledgments
Special thanks to Prof. Abdul-Kareem, Babylon University, Faculty of science for women, for his special care.
References
- Vashista, P.C. Taxonomy of Angiosperms.P.B.M. Press, New Delhi, India. Balunas.M.J.and A.D. Kinghorn, 2005. Drug discovery frommedicinalplants.LifeSci. 1974, 78, 431-441.
- Ogunti, E.O; Elujoba, A.A. Journal of Fitoterapia. 64 (5), 437-439.
- Al-Marzoqi, A.H.; Hameed, I.H.; Idan, S.A. African Journal of Biotechnology. 2015, 14(40), 2812-2830.
CrossRef - Hussein, A.O.; Mohammed, G.J.; Hadi, M.Y.; Hameed, I.H. Journal of Pharmacognosy and Phytotherapy. 2016, 8(3), 49-59.
CrossRef - Mishra, N.; Behal, K.K. Int. J Pharm Sci. 2010, 2, 187-196.
- Hussein, H.J.; Hadi, M.Y.; Hameed, I.H. 2016, 8(3), 60-89.
- Altameme, H.J.; Hadi, M.Y.; Hameed, I.H. Journal of Pharmacognosy and Phytotherapy. 2015a, 7(10), 238-252.
CrossRef - Al-Marzoqi, A.H.; Hadi, M.Y.; Hameed, I.H. Journal of Pharmacognosy and Phytotherapy. 2016, 8(2), 25-48.
CrossRef - Hadi, M.Y.; Mohammed, G.J.; Hameed, I.H. Journal of Pharmacognosy and Phytotherapy. 2016, 8(2), 8-24.
CrossRef - Martin, J.S.; Martin, M.M., Oecologia. 1982, 54, 205-211.
CrossRef - Winnicka, K.; Bielawski, K.; Bielawski, A. Acta Poloniae Pharmaceutica, 2006, 63, 109-115.
- PerwezHussain, S., Peijun, H.; Glewood, E. The Jour of Can Research. 2008, 68(17), 7130-6.
- Fabricant, D.S.; Farnsworth, N.R. Environ Health Prospect. 2001; 109: 69-75.
- Gupta, A.K.; Tondon, N.; Sharma, M. Quality Standards of Indian Medicinal Plants, Medicinal Plants Unit, Published by Indian Council of Medical Research, Vol 2, 2008, 47-53.
- Gupta, R.K. Medicinal & Aromatic plants, CBS publishers & distributors, 1st edition, 2010, 116-117.
- Kirtikar, K.R.; Basu, B.D. Indian Medicinal Plants, International book distributors, 2006, 2, 856-860.
- Padma, S. Indian Journal of Microbiology, 2006, 46(2), 169-170.
- Altameme, H.J.; Hameed, I.H.; Abu-Serag, N.A. Malays. Appl. Biol. 2015b, 44(4), 47–58.
- Hameed, I.H.; Abdulzahra, A.I.; Jebor, M.A.; Kqueen, C.Y.; Ommer, A.J. 2015a, 26(4), 544-9.
- Hameed, I.H.; Hamza, L.F.; Kamal, S.A. Journal of Pharmacognosy and Phytotherapy. 2015b, 7(8), 132-163.
CrossRef - Hussein, H.M.; Hameed, I.H.; Ibraheem, O.A. International Journal of Pharmacognosy and Phytochemical Research. 2016, 8(3).
- Altameme, H.J.; Hameed, I.H.; Idan, S.A.; Hadi, M.Y. Journal of Pharmacognosy and Phytotherapy. 2015c, 7(9), 221-237.
CrossRef - Sathyaprabha, G.; Kumaravel, S; Ruffina, D.; Praveenkumar, P.A. J Pharma Res. 2010, 3, 2970–2973.
- Hameed, I.H.; Hussein, H.J.; Kareem, M.A.; Hamad, N.S. Journal of Pharmacognosy and Phytotherapy. 2015c, 7 (7), 107-125.
CrossRef - Hamza, L.F.; Kamal, S.A.; Hameed, I.H. Journal of Pharmacognosy and Phytotherapy. 2015, 7(9), 194-220.
CrossRef - Jasim, H.; Hussein, A.O.; Hameed, I.H.; Kareem, M.A. Journal of Pharmacognosy and Phytotherapy. 2015, 7 (4), 56-72.
- Hameed, I.H.; Ibraheam, I.A.; Kadhim, H.J. Journal of Pharmacognosy and Phytotherapy. 2015d, 7(6), 90-106.
CrossRef - Udayaprakash, N.K.; Bhuvaneswari, S.; Jahnavi, B.; Abhinaya K.; Rajalin AG. Res J Med Plant. 2012, 6, 341-345.
CrossRef - Pallab, M.; Dhananjay H.; Uday B.; Dipak, K.M. Indian Journal of Experimental Biology, 2009, 47, 849-861.
- Chopra, R.N.; Nayar, S.L.; Chpora, I.C. Glossary of Indian Medicinal Plants, National Institute of Science Communication and Information Resources, 2006, page no. 54.
- Agarwal, S.S.; Paridhavi, M. Clinically useful herbal drugs, Ahuja Publishing House, 2005, 281-282.
- Kaur, R.; Kaur, H. Oriental Journal of Chemistry. 2015, 31(1), 597-600.
- Chandrashekharaiah, K.S. Oriental Journal of Chemistry. 2013, 29(3), 1061-1070.

This work is licensed under a Creative Commons Attribution 4.0 International License.









