Identification and Characterization of Solid Binary System of Quercetin-Nicotinamide
Erizal Zaini*, Dillah Azhari and Lili Fitriani
Faculty of Pharmacy, Andalas University, Kampus Limau Manis Padang, Indonesia 25163
Corresponding Author E-mail : erizal@ffarmasi.unand.ac.id
DOI : http://dx.doi.org/10.13005/ojc/320330
Article Received on : May 18, 2016
Article Accepted on : June 27, 2016
Preparation of binary system quercetin-nicotinamide and the solubility evaluation have been conducted in this study. Binary system was prepared by solvent evaporation technique and physical mixture was used as comparison. The aim of this study is to analyze the type of interaction and to investigate the solubility of quercetin-nicotinamide produced. Identification of binary system interaction were done using Powder X-ray diffraction (PXRD), Differential Thermal Analysis (DTA), Infrared spectroscopy (IR), Scanning Electron Microscopy (SEM), and solubility test. Determination of quercetin was done in ethanol:water (1:1) by High Performance Liquid Chromatography (HPLC) using acetonitrile:phosphate acid 0,1% (55:45) as mobile phases. Diffractogram powder X-ray showed a decrease in the peak intensity, but did not show a new crystalline phase. DTA thermal analysis showed a decrease in the melting point of the binary system compared to quercetin, which likely eutectic mixture was formed. SEM results indicated the changes in the morphology of the crystal compared to pure components. FT IR analysis showed a shift wavenumber of the spectrum quercetin and nicotinamide. Interaction between these compound showed that the conglomerated form (simple eutectic) between two crystalline phases. The solubility of binary system quercetin-nicotinamide increased compared to intact quercetin. Solubility of quercetin, physical mixture and binary system quercetin nicotinamide were 0.294±0.005 mg/mL, 0.338±0.004 mg/mL, and 0.419±0.001 mg/mL, respectively. In conclusion, the solubility increased after the formation of binary system quercetin-nicotinamide.
KEYWORDS:quercetin; nicotinamide; interaction; binary system; eutectic
Download this article as:| Copy the following to cite this article: Zaini E, Azhari D, Fitriani L. Identification and Characterization of Solid Binary System of Quercetin-Nicotinamide. Orient J Chem 2016;32(3). |
| Copy the following to cite this URL: Zaini E, Azhari D, Fitriani L. Identification and Characterization of Solid Binary System of Quercetin-Nicotinamide. Orient J Chem 2016;32(3). Available from: http://www.orientjchem.org/?p=17989 |
Introduction
Quercetin is a flavonoid polyphenol compound, which is still interesting to study due to several pharmacological activities1. However, quercetin has a low solubility in water, causing limitations in the process of absorption and effect on bioavailability in the body2. Several methods have been conducted to improve the solubility and dissolution rate of quercetin such as formation of inclusion complexes with cyclodextrins3, manufacture of solid dispersions1, and formation of nanoparticles4,5.
Efforts to improve the solubility of drug compounds which are poorly soluble in water generally involves the interaction between the two compounds (binary system) or more. Physical interaction of a binary system generally occurs in two resembled materials, which is generally based on the molecular formula, and the internal structure or the level of crystalline lattice symmetry6. Interactions are often found in pharmaceutical technology and a binary system can be classified into physical interaction eutectic system (conglomerate), peritecticum (solid solution), and molecular compounds (co-crystal)7.
Previous study showed that co-crystal quercetin can be formed by addition several co-former such as phenolic acid, lactamide and carnitine8. In this study nicotinamide is used as co-former to form binary system with quercetin. Nicotinamide is a safe and inert material which included as Generally Recognized as Safe (GRAS), thus it can be used in the development of pharmaceutical preparations9. Nicotinamide has been widely used as Cocrystal Coformer (CCF) for the formation of co-crystals with celecoxib10, theophylline11, ibuprofen12, carbamazepine13, lamotrigine14, and binary mixture of efavirenz15.
Therefore, the present study conducted formation of a binary system of quercetin with nicotinamide by solvent evaporation method. Identification and characterization of binary system formed were done by Power X-ray Diffraction, Differential Thermal Analysis (DTA), Scanning Electron Microcopy (SEM) analysis, and Infra-red spectroscopy analysis and solubility test.
Materials and Methods
Materials
Quercetin (Sigma Aldrich, Singapore), Nicotinamide (Kimia Farma, Indonesia), 96% ethanol (Brataco Chemika, Indonesia), methanol (Brataco Chemika, Indonesia), acetonitrile HPLC grade (Merck), ethanol pro analysis (Merck), phosphate acid and distilled water. All materials were used as received.
Preparation of binary system of Quercetin – Nicotinamide
The binary system was prepared by solvent evaporation method. Quercetin and nicotinamide was mixed with ratio 1: 1 (in equimolar) and diluted with ethanol until all materials dissolved. The solvent was evaporated by using a rotary evaporator, then stored in a sealed container in a desiccator.
Preparation of physical mixture of Quercetin – Nicotinamide
Physical mixture of quercetin – nicotinamide was prepared with ratio of 1: 1 (equimol). Prior to mixture process, both quercetin and nicotinamide were crushed in a mortar separately for 1 hour. The mixing process was done using a spatula and the mixture was stored in a desiccator.
Powder X-ray Diffraction Analysis
Powder X-ray diffraction analysis was conducted for quercetin, nicotinamide, binary system and physical mixture at room temperature using a diffractometer (PAN Analytical, The Netherlands). Samples was placed on a sample glass and leveled to prevent particle orientation during sample preparation. The measurement conditions were as follows: Cu metal targets, Kα filter, voltage 40 kV, a current of 40 mA, and analysis was carried out in the range of 2 theta 10 – 40º.
Differential Thermal Analysis
Thermal analysis of samples was carried out by using DTA apparatus (Shimadzu DTA / TG-60, Japan) which has been calibrated with Indium. Samples, about 3-5 mg, was placed on aluminum-covered plate. DTA apparatus was programmed in a temperature range of 30-330°C with a heating rate 10°C per minute. Thermal analysis was conducted for intact quercetin, intact nicotinamide, and physical mixture of quercetin-nicotinamide and binary system of quercetin-nicotinamide.
Scanning Electron Microscopy (SEM) Analysis
The morphology analysis was done for intact quercetin, intact nicotinamide, and physical mixture and binary system of quercetin-nicotinamide using an SEM apparatus (Jeol type JSM-6360LA, Japan). Powder sample was placed on a sample made of aluminum and coated with gold and the voltage was set at 20 kV and 12 mA current. The sample was then observed at different magnifications.
Infra-red spectroscopy analysis
The sample was placed on the ATR crystal to cover all surfaces of samples. Absorption spectra was recorded with Fourier Transform Infrared (FT-IR) at wavenumber 4000-500 cm-1. Analyses was performed for intact quercetin, intact nicotinamide, physical mixture and binary system of quercetin-nicotinamide.
Solubility test
Solubility test was conducted to measure the amount of quercetin dissolved in solvent used. An excessive amount sample of intact quercetin, physical mixture and binary system quercetin-nicotinamide was dissolved in ethanol: water (1:1). The solubility test was carried out using an orbital shaker for 24 hours. The sample was filtered using Whatman filter paper (0.45 µm) and filtrate solution was injected for HPLC analysis (Shimadzu LC-20AD) using acetonitrile:phosphate acid 0.1% (55:45) as mobile phases.
Results and Discussion
The results of X-ray diffraction analysis of intact quercetin, intact nicotinamide, and physical mixture of quercetin-nicotinamide and binary system of quercetin-nicotinamide is shown in Figure 1.
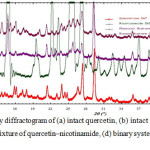 |
Figure 1: X-ray diffractogram of (a) intact quercetin, (b) intact nicotinamide, (c) physical mixture of quercetin–nicotinamide, (d) binary systems quercetin-nicotinamide Click here to View Figure |
It can be seen from diffractogram that there was a decrease in the intensity of interference binary system compared to intact quercetin. The decrease of intensity peak is likely due to solvent evaporation process which the recrystallization of crystal growth occurs resulting in the degree of crystallinity decreased. This indicated that the binary system between quercetin and nicotinamide did not produce a new crystalline phase (molecular compounds), but rather a conglomeration of two crystal phases in the solid state or often referred to as a simple eutectic mixture6.
Differential thermal analysis is used to evaluate changes in thermodynamic properties that occur when the material is given heat energy6. Changes in thermodynamic properties that occur when the material is given heat energy, including melting, recrystallization, desolvation and transformation into the solid phase can be observed by endothermic or exothermic peak on DTA thermogram. The result of differential thermal analysis (DTA) can be seen in Figure 2, which the melting point of intact quercetin, intact nicotinamide, physical mixture quercetin-nicotinamide, and binary system quercetin- nicotinamide was 318.1°C, 134.4°C, 231.3°C and 230.1°C, respectively.
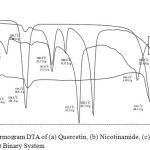 |
Figure 2: Thermogram DTA of (a) Quercetin, (b) Nicotinamide, (c) Physical Mixture, (d) Binary System Click here to View Figure |
There was a change in melting point for both physical mixture and binary system which likely indicated a physical interaction between quercetin and nicotinamide. The lower melting point of the binary system likely indicated formation of eutectic mixture between quercetin-nicotinamide15. Formation eutectic mixture has also been reported with efavirenz15 and coenzyme Q10 (Qo10)16. In addition, this result was in accordance with X-ray powder analysis which showed in diffractogram of quercetin-nicotinamide.
Morphology of intact quercetin, intact nicotinamide, physical mixture and binary system is shown in Figure 3.
 |
Figure 3: The scanning electron microscopy of: (a) intact quercetin, (b) intact nicotinamide, (c) physical mixture quercetin-nicotinamide, (d) binary system quercetin-nicotinamide. Click here to View Figure |
It can be seen that crystal habit of quercetin like needle shaped, while nicotinamide shaped like a rod. Moreover, the morphology of physical mixture was a combination of quercetin and nicotinamide, while the morphology of binary system was different in which the crystal size reduced and formed aggregate compared to both intact quercetin and nicotinamide. This indicated interaction has been occurred between quercetin and nicotinamide as also shown in thermal analysis that affect the crystal morphology of binary system.
Infra-red spectroscopy analysis was conducted to observe the shift on spectrum that can be used to identify a sample by comparing its spectrum to standard spectrum and to determine the interaction between the drugs with other substances in the sample. The infra-red spectrum of intact quercetin, intact nicotinamide, physical mixture and binary system can be seen in Figure 4.
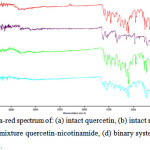 |
Figure 4: Infra-red spectrum of: (a) intact quercetin, (b) intact nicotinamide, (c) physical mixture quercetin-nicotinamide, (d) binary system quercetin-nicotinamide. Click here to View Figure |
The spectrum of intact quercetin was relatively similar to spectrum quercetin in literature at 2000-400 cm-1 wavelength. At wavelength 1655 cm-1, quercetin spectrum showed a stretching frequency of C=O bonding that normally showed at 1800 – 1600 cm-1. Nicotinamide spectrum showed a stretching of N-H bonding at 3357.96 cm-1 wavelength that appeared at 3060-3500 cm-1 wavelength. The spectrum of binary system showed difference in sharpness of peak appeared. There was a shift of C=O bonding at 1686 cm-1 compared to intact quercetin at 1655 cm-1 wavelength. Wavenumber shift that occurred likely due to the formation of a hydrogen bond between the carbonyl group of the amide groups on the quercetin with nicotinamide17.
The solubility test was conducted in ethanol and water medium due to properties of quercetin that can undergo oxidation in the water and oxygen in water and air18. The result of solubility test of intact quercetin, physical mixture and binary system can be seen in Table 1.
Table 1: Solubility of intact quercetin, physical mixture and binary system
| Sample | Solubility (mg/mL) |
| Intact quercetin | 0.294±0.005 |
| Physical mixture | 0.338±0.004 |
| Binary system | 0.419±0.001 |
The solubility of intact quercetin was 0.297±0.005 mg/mL which was similar to another study2. There was an increase in solubility for both physical mixture and binary system. This improvement was likely due to several mechanisms including addition of solvent to disperse quercetin molecularly and formation of eutectic that reduced particle size6. Moreover, the chemical interaction that likely occurred between quercetin and nicotinamide increased the solubility due to properties of nicotinamide which is highly hydrotropic causing an increase in solubility of quercetin19. The solubility improvement result was corresponded to DTA result, which a decrease in the melting point of the binary system formed. The decline was expected to cause the solubility of binary system higher than intact quercetin which has higher melting point. The lower the melting point of a compound the faster this compound to be dissolved.
Conclusion
The characterization and identification of binary system between quercetin and nicotinamide by X-ray diffraction, DTA, FT-IR, and SEM showed the formation of a simple eutectic mixture. The binary system exhibited the highest solubility compared to intact quercetin and physical mixture.
References
- Zhu, J.; Yang, Z.G.; Chen, X.M.; Sun, J.B.; Awuti, G.; Zhang, X. & Zhang, Q. Journal of Chinese Pharmaceutical Science, 2007, 16, 51-56
- Smith, A.J.; Kavuru, P.; Wojtas, L.; Zaworotko, M.J.; Shytle, R.D. Mol. Pharm.., 2011, 8(5):1867-76.
CrossRef - Borghetti, G.S.; Lula, I.S.; Sinisterra, R.D.; Bassani, V.L. AAPS Pharm. Sci. Tech. 2009, 10(1):235-42.
CrossRef - Wu, T.H.; Yen, F.L.; Lin, L.T.; Tsai, T.R.; Lin, C.C.; Cham, T.M. Int J. Pharm. 2008, 346, 160-168
CrossRef - Dhawan, S.; Kapil, R.; Singh, B. J. Pharm. and Pharmacol. 2011, 63(3), 342-51.
CrossRef - Zaini, E.; Sumirtapura, Y.C.; Soewandhi, S. N.; Halim, A.; Uekusa, H.; Fujii, K. Asian J. Pharm. Clin. Res., 2010, 3, 26-29.
- Davis, R.E.; Lorimer, K.A.; Wilkowski, M.A.; Rivers, J.H. ACA Transactions. 2004, 39, 41-61.
- Veverka, M.; Dubaj, T.; Gallovič, J.; Jorík, V.; Veverková, E.; Danihelová, M.; Šimon, P. Monatshefte für Chemie-Chemical Monthly., 2015, 146(1), 99-109.
CrossRef - Lemmerer, A.; Bernstein, J. Cryst.Eng. Comm., 2010, 12(7), 2029-33.
CrossRef - Remenar, J.F.; Peterson, M.L.; Stephens, P.W.; Zhang, Z.; Zimenkov, Y.; Hickey, M.B. Mol. Pharm. 2007, 4(3), 386-400.Lu, J.; Rohani, S. Org. Process Res. Dev. 2009, 13(6), 1269-75.
- Chow, S.F.; Chen, M.; Shi, L.; Chow, A.H.; Sun, C.C. Pharm. Res, 2012, 29(7), 1854-65.
CrossRef - Chieng, N.; Hubert, M.; Saville, D.; Rades, T.; Aaltonen, J. Cryst. Growth Des. 2009, 9(5), 2377-86.
CrossRef - Cheney, M.L.; Shan, N.; Healey, E.R.; Hanna, M.; Wojtas, L.; Zaworotko, M.J.; Sava. V; Song. S; Sanchez-Ramos, J.R. Cryst. Growth Des., 2009, 10(1), 394-405.
CrossRef - Zaini, E.; Rachmaini, F.; Armin, F.; Fitriani, L. Orient. J. Chem. 2015, 31(4), 2271-6.
CrossRef - Tarate, B.; Bansal, A.K. Thermochim. Acta. 2015, 605, 100-6.
CrossRef - Mirza, S.; Miroshnyk, I.; Heinämäki, J.; Yliruusi, J. Dosis. 2008, 24(2), 90-6.
- Zenkevich, I.G.; Eshchenko, A.Y.; Makarova, S.V.; Vitenberg, A.G.; Dobryakov, Y.G.; Utsal, V.A. Molecules. 2007, 12(3), 654-72.
CrossRef - Tripathy, S.; Kar, P.K. Orient J. Chem. 2013, 29(3), 1103-1109

This work is licensed under a Creative Commons Attribution 4.0 International License.









