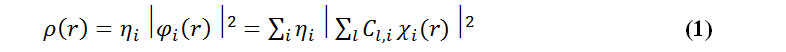First Principal and QM/MM Study of Dopamine Adsorption on Single Wall Carbon Nano Tubes and Single Wall Boroan Nitride Nano Tubes
Mojgan Jalili
Department of Chemistry, Science and Research Branch, Islamic Azad University, Tehran, Iran.
Corresponding Author Email : mojganjalili82@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/320335
Article Received on : March 29, 2016
Article Accepted on : May 03, 2016
Article Published : 19 May 2016
In the brain, dopamine functions as a neurotransmitter a chemical released by neurons (nerve cells) to send signals to other nerve cells. The brain includes several distinct dopamine pathways, one of which plays a major role in reward-motivated behavior. In this work we have calculated our systems based on the Dopamine binding with various diameters of SWBNNTs and SWCNTs. In this work, the electron density profile in the composition of the Dopamine binding to SWCNTs and SWBNNTs have been calculated.
KEYWORDS:dopamine; adsorption on SWCNTs and SWBNNTs; LOL; ELF
Download this article as:| Copy the following to cite this article: Jalili M. First Principal and QM/MM Study of Dopamine Adsorption on Single Wall Carbon Nano Tubes and SingleWall Boroan Nitride Nano Tubes. Orient J Chem 2016;32(3). |
| Copy the following to cite this URL: Jalili M. First Principal and QM/MM Study of Dopamine Adsorption on Single Wall Carbon Nano Tubes and SingleWall Boroan Nitride Nano Tubes. Orient J Chem 2016;32(3). Available from: http://www.orientjchem.org/?p=16144 |
Introduction
Dopamine is an organic chemical of the catecholamine and phenethylamine families that plays several important roles in the brain and body. it is an amine synthesized by removing a carboxyl group from a molecule of its precursor chemical L-DOPA, which is synthesized in the brain and kidneys. Dopamine is also synthesized in plants and most multicellular animals.
In the brain, dopamine functions as a neurotransmitter a chemical released by neurons (nerve cells) to send signals to other nerve cells. The brain includes several distinct dopamine pathways, one of which plays a major role in reward-motivated behavior. Most types of reward increase the level of dopamine in the brain, and most addictive drugs increase dopamine neuronal activity. Other brain dopamine pathways are involved in motor control and in controlling the release of various hormones.
Outside the central nervous system, dopamine functions in several parts of the peripheral nervous system as a local chemical messenger. Several important diseases of the nervous system are associated with dysfunctions of the dopamine system, and some of the key medications used to treat them work by altering the effects of dopamine. Parkinson’s disease, a degenerative condition causing tremor and motor impairment, is caused by a loss of dopamine-secreting neurons in an area of the midbrain (called the substantia nigra).
Its metabolic precursor L-DOPA can be manufactured, and in its pure form marketed as Levodopa is the most widely used treatment for the condition. There is evidence that schizophrenia involves altered levels of dopamine activity, and most antipsychotic drugs used to treat this are dopamine antagonists which reduce dopamine activity. Similar dopamine antagonist drugs are also some of the most effective anti-nausea agents. Restless legs syndrome and attention deficit hyperactivity disorder (ADHD) are associated with decreased dopamine activity. Dopaminergic stimulants can be addictive in high doses, but some are used at lower doses to treat ADHD. Dopamine itself is available as a manufactured medication for intravenous injection: although it cannot reach the brain from the bloodstream, its peripheral effects make it useful in the treatment of heart failure or shock, especially in newborn babies [1,2].
The carbon nanotube (CNT) is a representative nano-material. CNT is a cylindrically shaped carbon material with a nano-metric-level diameter.
Carbon nano cone has a high asymmetric geometry that in our simulations, classical non-equilibrium molecular dynamics method is adopted. The cone is entirely characterized by its cone angle. When one pentagon is introduced into a hexagonal carbon network, a 60 declination defect is formed; leading to the formation of a nano cone with cone angle of 118 and the equilibrium carbon-carbon bond length is 1.418 Å. In this work, we focus on the cone with the cone angle of 180, which is the largest angle observed experimentally and theoretically.
Moreover, in all theoretical models so far, the rectification efficiency decreases quickly as the structure length increases Its structure, which is in the form of a hexagonal mesh, resembles a graphite sheet and it carries a carbon atom located on the vertex of each mesh. The sheet has rolled and its two edges have connected seamlessly.
Although it is a commonplace material using in pencil leads, its unique structure causes it to present characteristics that had not found with any other materials. CNT can be classified into single-wall CNT, double-wall CNT and multi-wall CNT according to the number of layers of the rolled graphite.
The type attracting most attention is the single-wall CNT, which has a diameter deserving the name of “nanotube” of 0.4 to 2 nanometers. The length is usually in the order of microns, but single-wall CNT with a length in the order of centimeters has recently released
CNT can be classified into single-wall CNT, double-wall CNT and multi-wall CNT according to the number of layers of the rolled graphite. The type attracting most attention is the single-wall CNT, which has a diameter deserving the name of “nanotube” of 0.4 to 2 nanometers
The length is usually in the order of microns, but single-wall CNT with a length about centimeters have recently released. The extremities of the CNT have usually closed with lids of the graphite sheet
Boron nitride nanotube (BNNTs) has attracted many interests due to their large gap semi conducting character. Boron nitride (BN) is a structural existing in cubic (diamond-like), hexagonal (graphite-like), turbo static, and amorphous forms .these compounds have been produced by a variety of methods, such as arc melting, high temperature chemical reaction, carbon nanotube templates, and laser ablating, The most attention has been focused on the development of new methods for the production of nanotube and inorganic fullerene of other materials.
In addition, theoretical calculations have been described the possible existence of small BN clusters.
Theoretical studies have been performed for BN doped in CNTs which it has been found that a structure built from squares and hexagons is more stable than those built from pentagons and hexagons. This is because in the second case less stable B-B and N-N bonds are formed.
Monajjemi and coworkers have simulated a large range of the nanotube carbons and other molecules via a wide range of methods and basis sets in the field of Physical chemistry, Nano biotechnology, quantum phenomenon, Nano capacitors, chemical biology, adsorption, and NMR shielding [3-119].
In this work we have calculated our systems based on the dopamine binding with various diameters of SWBNNTs and SWCNTs .The effects of these binding for the drug delivery can also be calculated for any further comparison and discussion.
Zigzag BNNTs (n, 0) are expected to have direct band gap. On the other hand, armchair BNNTs (m, m) will have indirect band gap [120]. Because of their large band gap of (~5 eV),
experiments using BNNTs as the conduction channel for field-effect transistors (FETs) showed that BNNTs allowed transport through only the valence band.
Another important feature about the band gaps of BNNTs is that they are tunable by doping with carbon , radial deformation ,or by applying a transverse electric field across the BNNT “so-called giant stark effect” [121].
Electron localization function (ELF)
In this work, the electron density profile in the composition of the Dopamine binding to SWCNTs and SWBNNTs have been calculated.
The electron density has been defined as:
where is occupation number of orbital i, is orbital wave function, c is the basis function, C is
Bader [122] has found that the regions with large electron localization must have large
magnitudes of Fermi-hole integration. However, the Fermi hole is a six-dimension function and
thus difficult to be studied visually. Becke and Edgecombe noted that spherically averaged like spin conditional pair probability has a direct correlation with the Fermi hole and further suggested electron localization function (ELF) as:
Savin et al. have reinterpreted ELF in the viewpoint of kinetic energy, [123] which would make ELF also meaningful for Kohn-Sham DFT wave-function or even post-HF wave-function. They indicated that D(r) reveals the excess kinetic energy density caused by Pauli repulsion, while D0(r) can be considered as Thomas-Fermi kinetic energy density.
To overcome this problem, Multiwfn automatically adds a minimal value of 10-5 to D(r). This treatment does not really affect the ELF value in interesting regions, in which case, the actual kinetic energy term in D(r) is replaced by Kirzhnits type second-order gradient expansion that is:

where
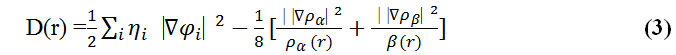
and
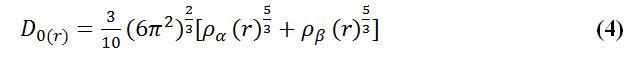
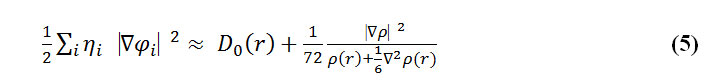
So that ELF is totally independent of the wave-function and can be used to analyze electron density from X-ray diffraction data. It is clear that Tsirelson’s ELF can also be used to analyze electron density from quantum chemistry calculation, though, it is not as good as the ELF defined by Becke owing its suitability to the approximation introduced in kinetic energy term; however, qualitative conclusions can still be recovered in general.
Another function similar to ELF is called the localized orbital locator (LOL) which is used to locate high localization regions.
LOL contains a similar expression compared to ELF as well. In fact, the chemically significant regions that highlighted by LOL and ELF are generally qualitatively comparable, while Jacobsen pointed out that LOL conveys clearer and more decisive picture compared to ELF, [123-127]. Clearly, LOL can be interpreted in terms of kinetic energy similar to ELF. However, LOL can also be interpreted in terms of localized orbital. Small or large value of LOL is usually appeared in boundary (inner) region of localized orbitals due to the large of small gradient of orbital wave-function in this area respectively. The value range of LOL is identical to ELF, namely [0, 1].
Computational Details
Parts of the Nanotubes including dopamine have been modeled with the QM/MM method and the calculations are carried out with the Monte Carlo method.
In this investigation, differences in force field are illustrated by comparing the calculated energy with AMBER and OPLS force fields. Furthermore, a HyperChem professional release 7.01 programs is used for the additional calculations.
The final parameterization of nanotubes was computed using self-consistent field calculations in order to find the optimal starting geometry, as well as the partial charges. The density functional theory with the van der Waals density functional was employed to model the exchange-correlation energies of graphene layers. All optimization of graphene layers were performed by Gaussian 09. The main focus in this study is to obtain the results from DFT methods such as m062x, m06-L, and m06 for the methamphetamine and dopamine. The m062x, m06-L and m06-HF are rather new DFT functional with a good correspondence in adsorption calculations between tubes and methamphetamine and dopamine are useful for the energies and other physical chemistry data.
Geometry optimizations and electronic structure calculations have been carried out using the m06 (DFT) functional. This approach is based on an iterative solution of the Kohn-Sham equation [128] of the density functional theory in a plane-wave set with the projector-augmented wave pseudo-potentials. The Perdew-Burke-Ernzerhof (PBE) [129] exchange-correlation (XC) functional of the generalized gradient approximation (GGA) is also used. The optimizations of the lattice constants and the atomic coordinates are made by the minimization of the total energy.
The charge transfer and electrostatic potential-derived charge were also calculated using the Merz-Kollman-Singh [130], chelp [131], or chelpG [132].
Result and Discussion
In this study, difference in force field is illustrated by comparing the energy calculated by using force fields, MM+ and OPLS. Also, it has been investigated polar solvent and the temperature effects (between 260K and 400K) on the stability of SWBNNT bonded to methamphetamine and dopamine in various solvents. The quantum mechanics (QM) calculations were carried out with the HyperChem 8.0 program. This study mainly focuses on the electron density of methamphetamine and dopamine in a binding system with (n, n) SWBNNTs surfaces and SWCNTs. The models and situation of molecular structures and binding interaction are shown in figs1- 5. As it is indicated in table HOMO/LUMO and Gap energy shows suitable binding [133,134].
According to the above equations the largest electron localization is located on atoms which are bonded to nanotubes where the electron motion is more likely to be confined within that region. If electrons are completely localized in those atoms, they can be distinguished from the ones outside. As shown in figures1-45 the large density is close to the bonded atoms. The regions with large electron localization need to have large magnitudes of Fermi-hole integration which would lead those atoms towards superparamagnetic. The fermi hole is a six-dimension function and as a result, it is difficult to be studied visually. Based those equations, Becke and Edgecombe noted that the Fermi hole is a spherical average of the spin which is in good agreement with our results in tables and Figs .
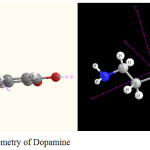 |
Figure 1: Molecular geometry of Dopamine Click here to View figure |
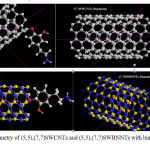 |
Figure 2: Molecular geometry of (5,5),(7,7)SWCNTs and (5,5),(7,7)SWBNNTs with binding to Dopamine Click here to View figure |
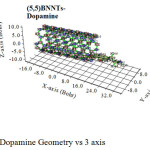 |
Figure 3: Dopamine Geometry vs 3 axis Click here to View figure |
 |
Table 1: HOMO, LUMO and BAND GAP of some Dopamine-(5, 5) SWBNNT Click here to View table |
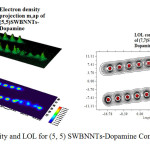 |
Figure 4: Electron density and LOL for (5, 5) SWBNNTs-Dopamine Complex Click here to View figure |
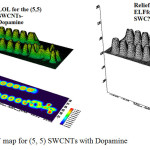 |
Figure 5: LOL and ELF map for (5, 5) SWCNTs with Dopamine Click here to View figure |
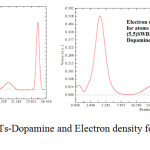 |
Figure 6: G ( r ) for (7,7) SWCNTs-Dopamine and Electron density for (5,5)SWBNNTs Complexes Click here to View figure |
References
- Dopamine: Biological activity”. IUPHAR/BPS guide to pharmacology. International Union of Basic and Clinical Pharmacology. Retrieved 29 January (2016).
- Volkow, ND.; Wang, GJ.; Kollins, SH.; Wigal, TL.; Newcorn, JH.;Telang, F.; Fowler, JS.; Zhu, W.; Logan, J.; Ma, Y.; Pradhan, K.; Wong, C.; Swanson, JM, JAMA (2009) 302 (10): 1084–91. doi:10.1001/jama.2009.1308. PMC 2958516. PMID 19738093.
- Monajjemi, M.; Lee, V.S.; Khaleghian, M.; B. Honarparvar, B.; F. Mollaamin, F. J. Phys.Chem C. 2010, 114, 15315
- Monajjemi, M. Struct Chem. 2012, 23,551–580
- Monajjemi, M.; Chegini, H.; Mollaamin, F.; Farahani, P. Fullerenes, Nanotubes, and Carbon Nanostructures. 2011, 19, 469–482
- Monajjemi, M .; Afsharnezhad ,S.; Jaafari , M.R.; Abdolahi ,T.; Nikosade ,A.; Monajemi ,H.; Russian Journal of physical chemistry A, 2007, 2,1956-1963
- Monajjemi, M.; Baei, M.T.; Mollaamin, F. Russian Journal of Inorganic Chemistry. 2008, 53 (9), 1430-1437
- Monajjemi, M.; Rajaeian, E.; Mollaamin, F.; Naderi, F.; Saki, S. Physics and Chemistry of Liquids. 2008, 46 (3), 299-306
- Monajjemi, M.; Boggs, J.E. J. Phys. Chem. A, 2013, 117, 1670 −1684
- Mollaamin, F.; Monajjemi, M, Journal of Computational and Theoretical Nanoscience. 2012, 9 (4) 597-601
- Monajjemi, M.; Khaleghian, M, Journal of Cluster Science. 2011, 22 (4), 673-692
- Mollaamin, F.; Varmaghani, Z.; Monajjemi, M, Physics and Chemistry of Liquids. 2011, 49 318
- Nafisi, S.; Monajemi, M.; Ebrahimi, S. Journal of Molecular Structure. 2004,705 (3) 35-39
- Fazaeli, R.; Monajjemi, M.; Ataherian, F.; Zare, K. Journal of Molecular Structure: THEOCHEM.2002, 581 (1), 51-58
- Monajjemi, M.; Razavian, M.H.; Mollaamin,F.; Naderi,F.; Honarparvar,B.; Russian Journal of Physical Chemistry A , 2008 , 82 (13), 2277-2285
- Monajjemi, M.; Seyed Hosseini, M.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures. 2013, 21, 381–393
- Monajjemi, M.; Faham, R.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures, 2012 20, 163–169
- Mollaamin, F.; Najafi, F.; Khaleghian, M.; Khalili Hadad, B.; Monajjemi, M. Fullerenes, Nanotubes, and Carbon Nanostructures, 2011 19, 653–667
- Mollaamin, F.; Baei, MT.; Monajjemi, M.; Zhiani, R.; Honarparvar, B.;
- Russian Journal of Physical Chemistry A, Focus on Chemistry, 2008, 82 (13), 2354-2361
- Monajjemi, M. Chemical Physics. 2013, 425, 29-45
- Monajjemi, M.; Heshmat, M.; Aghaei, H.; Ahmadi, R.; Zare, K. Bulletin of the Chemical Society of Ethiopia, 2007, 21 (1)
- Monajjemi, M.; Honarparvar, B. H. ; Haeri, H. ; Heshmat ,M.; Russian Journal of Physical Chemistry C. 2006, 80(1):S40-S44
- Monajjemi, M.; Ketabi, S.; Amiri, A. Russian Journal of Physical Chemistry, 2006, 80 (1), S55-S62
- Yahyaei, H.; Monajjemi, M.; Aghaie, H.; K. Zare, K. Journal of Computational and Theoretical Nanoscience. 2013, 10, 10, 2332–2341
- Mollaamin, F.; Gharibe, S.; Monajjemi, M. Int. J. Phy. Sci, 2011, 6, 1496-1500
- Monajjemi, M.; Ghiasi, R.; Seyed Sadjadi, M.A. Applied Organometallic Chemistry,2003, 17, 8, 635–640
- Monajjemi, M.; Wayne Jr, Robert. Boggs, J.E. Chemical Physics. 2014, 433, 1-11
- Monajjemi, M.; Sobhanmanesh, A.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures, 2013, 21 47–63
- Monajjemi, M.; Mollaamin, F. Journal of Computational and Theoretical Nanoscience, 2012, 9 (12) 2208-2214
- Monajjemi, M.; Honarparvar, B.; Nasseri, S. M. .; Khaleghian M. Journal of Structural Chemistry. 2009, 50, 1, 67-77
- Monajjemi, M.; Aghaie, H.; Naderi, F. Biochemistry (Moscow).2007, 72 (6), 652-657
- Ardalan, T.; Ardalan, P.; Monajjemi, M. Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22: 687–708
- Mollaamin, F.; Monajjemi, M.; Mehrzad, J. Fullerenes, Nanotubes, and Carbon Nanostructures. 2014, 22: 738–751
- Monajjemi, M.; Najafpour, J.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures. 2013, 21(3), 213–232
- Monajjemi, M.; Karachi, N.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22: 643–662
- Yahyaei, H.; Monajjemi, M. Fullerenes, Nanotubes, and Carbon Nanostructures.2014, 22(4), 346–361
- Monajjemi, M. Falahati, M.; Mollaamin, F.; Ionics, 2013, 19, 155–164
- Monajjemi, M.; Mollaamin, F. Journal of Cluster Science, 2012, 23(2), 259-272
- Tahan, A.; Monajjemi, M. Acta Biotheor, 2011, 59, 291–312
- Lee, V.S.; Nimmanpipug, P.; Mollaamin, F.; Kungwan, N.; Thanasanvorakun, S..; Monajjemi, M. Russian Journal of Physical Chemistry A, 2009, 83, 13, 2288–2296
- Monajjemi, M.; Heshmat, M.; Haeri, HH, Biochemistry (Moscow), 2006, 71 (1), S113-S122
- Monajjemi, M.; Yamola, H.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22, 595–603
- Mollaamin, F.; Layali, I.; Ilkhani A. R.; Monajjemi, M. African Journal of Microbiology Research .2010, 4(24) 2795-2803
- Mollaamin, F.; Shahani poor, p K. .; Nejadsattari, T. ; Monajjemi, M. African Journal of Microbiology Research. 2010, 4(20) 2098-2108
- Monajjemi, M.; Ahmadianarog, M. Journal of Computational and Theoretical Nanoscience. 2014, 11(6), 1465-1471
- Monajjemi, M.; Jafari Azan, M.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures.2013, 21(6), 503–515
- Mollaamin, F.; Monajjemi, M. Physics and Chemistry of Liquids .2012, 50, 5, 2012, 596–604
- Monajjemi, M.; Khosravi, M.; Honarparvar, B.; Mollaamin, F.; International Journal of Quantum Chemistry, 2011, 111, 2771–2777
- Khaleghian, M.; Zahmatkesh, M.; Mollaamin, F.; Monajjemi, M. Fullerenes, Nanotubes, and Carbon Nanostructures, 2011, 19(4): 251–261
- Monajjemi, M.; Baheri, H.; Mollaamin, F. Journal of Structural Chemistry.2011 52(1), 54-59
- Mahdavian, L.; Monajjemi, M.; Mangkorntong, N. Fullerenes, Nanotubes and Carbon Nanostructures, 2009, 17 (5), 484-495
- Monajjemi, M., Mahdavian, L., Mollaamin, F. Bull. Chem. Soc. Ethiop. 2008, 22(2), 277-286
- Monajjemi, M.; Afsharnezhad, S, Jaafari, M.R..; Mirdamadi, S..; Mollaamin, F..; Monajemi, H. Chemistry .2008, 17 (1), 55-69
- Monajjemi, M.; Mollaamin, F.; Gholami, M. R.; Yoozbashizadeh, H.; Sadrnezhaad, S.K.; Passdar, H.; Main Group Metal Chemistry, 2003, 26, 6, 349-361
- Monajjemi, M.; Azad ,MT.; Haeri, HH.; Zare, K.; Hamedani, Sh.; JOURNAL OF CHEMICAL RESEARCH-S.2003, (8): 454-456
- Monajjemi, M.; Najafpour, J. Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22(6): 575–594
- Monajjemi, M.; Noei, M.; Mollaamin, F. Nucleosides, Nucleotides and Nucleic Acids. 2010 29(9):676–683
- Ghiasi, R.; Monajjemi, M. Journal of Sulfur Chemistry .2007,28, 5, 505-511
- Monajjemi, M.; Ghiasi, R.; Abedi, A. Russian Journal of Inorganic Chemistry.2005, 50(3), 382-388
- Monajjemi, M. .; Naderi, F.; Mollaamin, F.; Khaleghian, M. J. Mex. Chem. Soc. 2012, 56(2), 207-211
- Monajjemi, M.; Farahani, N.; Mollaamin, F. Physics and Chemistry of Liquids, 2012, 50(2) 161–172
- Monajjemi, M.; Seyed Hosseini, M. Journal of Computational and Theoretical Nanoscience .2013, 10 (10), 2473-2477
- Monajjemi , M.; Honaparvar , B.; Khalili Hadad ,B.; Ilkhani ,AR.; Mollaamin, F. African Journal of Pharmacy and Pharmacology .2010, 4(8), 521 -529
- Monajjemi, M. Theor Chem Acc, 2015, 134:77 DOI 10.1007/s00214-015-1668-9
- Monajjemi, M. Journal of Molecular Modeling , 2014, 20, 2507
- Monajjemi , M.; Honarparvar, B.; Monajemi, H.;. Journal of the Mexican Chemical Society, 2006, 50 (4), 143-148
- Monajjemi, M.; Khaleghian, M.; Mollaamin, F. Molecular Simulation. 2010, 36, 11, 865–
- Ilkhani, Ali R.; Monajjemi, M. Computational and Theoretical Chemistry.2015 1074, 19–25
- Monajjemi, M. Biophysical Chemistry. 2015 207,114 –127
- Monajjemi, M., Moniri, E., Panahi, H.A , Journal of Chemical and Engineering Data.2001, 1249-1254.
- Mollaamin, F.; Najafpour, J.; Ghadami, S.; Ilkhani, A. R.; Akrami, M. S.; Monajjemi, M. Journal of Computational and Theoretical Nanoscience. 11 (5), 1290-1298
- Monajjemi, M.; Ghiasi, R.; Ketabi, S.; Passdar, H.; Mollaamin, F. Journal of Chemical Research . 2004, 1, 11.
- Monajjemi, M.; Heshmat, M.; Haeri, H.H. Biochemistry (Moscow).2006, 71, 113-122
- Monajjemi, M.; Heshmat, M.; Aghaei, H.;Ahmadi, R.; Zare, K. Bulletin of the Chemical Society of Ethiopia. 2007, 21, 111–116
- Monajjemi, M., Kharghanian, L., Khaleghian, M., Chegini, H. Fullerenes Nanotubes and Carbon Nanostructures.2014, 22, 8, 0.1080/1536383X.2012.717563
- Sarasia, E.M.; Afsharnezhad, S.; Honarparvar, B.; Mollaamin, F.; Monajjemi, M.
- Physics and Chemistry of Liquids. 2011, 49 (5), 561-571
- Amiri, A.; Babaeie, F.; Monajjemi, M. Physics and Chemistry of Liquids. 2008, 46, 4, 379-389
- Monajjemi, M.; Heshmat, M.; Haeri, H.H.; Kaveh, F. Russian Journal of Physical Chemistry A, 2006, 80, 7, 1061-1068
- Monajjemi, M.; Moniri, E.; Azizi, Z.; Ahmad Panahi, H. Russian Journal of Inorganic Chemistry. 2005, 50, 1, 40-44
- Jalilian,H.; Monajjemi, M. Japanese Journal of Applied Physics. 2015, 54, 8, 08510
- Mollaamin, F.; Monajjemi, M. Journal of Computational and Theoretical Nanoscience. 2015, 12, 6, 1030-1039
- Felegari, Z.; Monajjemi, M. Journal of Theoretical and Computational Chemistry. 2015, 14, 3, 1550021
- Shojaee, S., Monajjemi, M. Journal of Computational and Theoretical Nanoscience. 2015, 12, 3, 449-458
- Esmkhani, R.; Monajjemi, M. Journal of Computational and Theoretical Nanoscience. 2015. 12, 4, 652-659
- Monajjemi, M., Seyedhosseini, M., Mousavi, M., Jamali, Z. Journal of Computational and Theoretical Nanoscience. 2015 , 23 (3), 239-244
- Ghiasi, R.; Monajjemi, M.; Mokarram, E.E.; Makkipour, P. Journal of Structural Chemistry. 2008, 4 , 4, 600-605
- . Mahdavian, L.; Monajjemi, M. Microelectronics Journal. 2010, 41(2-3), 142-149
- Monajjemi, M.; Baie, M.T.; Mollaamin, F. Russian Chemical Bulletin.2010, 59, 5, 886-889
- Bakhshi, K.; Mollaamin, F.; Monajjemi, M. Journal of Computational and Theoretical Nanoscience. 2011, 8, 4, 763-768
- Darouie, M.; Afshar, S.; Zare, K., Monajjemi, M. journal of Experimental Nanoscience.2013, 8, 4, 451-461
- Amiri, A.; Monajjemi, M.; Zare, K.; Ketabi, S. Physics and Chemistry of Liquids. 2006, 44, 4, 449-456.
- Zonouzi, R.;Khajeh, K.; Monajjemi, M.; Ghaemi, N. Journal of Microbiology and Biotechnology. 2013, 23, 1 ,7-14
- Ali R. Ilkhani .; Majid Monajjemi, Computational and Theoretical Chemistry.2015, 1074 19–25
- Tahan, A.; Mollaamin, F.; Monajjemi, M. Russian Journal of Physical Chemistry A, 2009, 83 (4), 587-597
- Khalili Hadad, B.; Mollaamin, F.; Monajjemi, M, Russian Chemical Bulletin,2011, 60(2):233-236
- Mollaamin, F.; Monajjemi, M.; Salemi, S.; Baei, M.T. Fullerenes Nanotubes and Carbon Nanostructures, 2011, 19, 3, 182-196
- Mollaamin, F.; Shahani Pour.; K., Shahani Pour, K.; ilkhani, A.R.; Sheckari, Z., Monajjemi, M Russian Chemical Bulletin , 2012 , 61(12), 2193-2198
- Shoaei, S.M.; Aghaei, H.; Monajjemi, M.; Aghaei, M. Phosphorus, Sulfur and Silicon and the Related Elements. 2014, 189, 5; 652-660
- Mehrzad, J., Monajjemi, M., Hashemi, M , Biochemistry (Moscow).2014 , 79 (1), 31-36
- Moghaddam, N.A., Zadeh, M.S., Monajjemi, M. Journal of Computational and Theoretical Nanoscience , 2015 , 12, 3, doi:10.1166/jctn.2015.3736
- Joohari, S.; Monajjemi, M , Songklanakarin Journal of Science and Technology , 2015 , 37(3):327
- Rajaian, E., Monajjemi, M., Gholami, M.R, Journal of Chemical Research – Part S ,2002, 6, 1, 279-281
- Ghassemzadeh, L., Monajjemi, M., Zare, K , Journal of Chemical Research – Part S, 2003, 4, 195-199
- Barforushi. M. M., Safari. S., and Monajjemi. M. ., J. Comput. Theor. Nanosci. 2015,12, 3058-3065
- Mollaamin. F., Ilkhani. A., Sakhaei. N., Bonsakhteh. B., Faridchehr. A., Tohidi. S., and Monajjemi. M. ., J. Comput. Theor. Nanosci. 2015,12, 3148-3154
- Rahmati. H. and Monajjemi. M J. Comput. Theor. Nanosci. 2015.,12, 3473-3481
- Bashiz. R. T. ., and Monajjemi. M .,J. Comput. Theor. Nanosci. 2015.,12, 3808-3816
- Nejad. A.M. and Monajjemi. M .,J. Comput. Theor. Nanosci. 2015.,12, 3902-3910
- Monajjemi. M, Bagheri., Moosavi M. S., Moradiyeh. N., Zakeri. M.,
- Attarikhasraghi. N., Saghayimarouf. N., Niyatzadeh. G., Shekarkhand. M.,
- Khalilimofrad. M.S., Ahmadin. H. and Ahadi. M., Molecules 2015, 20, 21636–21657; doi:10.3390/molecules201219769
- Shabanzadeh. E. and Monajjemi. M., J. Comput. Theor. Nanosci. 2015., 12, 4076-4086
- Elsagh. A., Jalilian. H., Kianpour. E., Mokri. H. S. G., Rajabzadeh. M., Moosavi M. S., Amiri. F. G., and Monajjemi. M., J. Comput. Theor. Nanosci. 2015.,12, 4211-4218
- Faridchehr. A., Rustaiyan. A., and Monajjemi. M. J. Comput. Theor. Nanosci. 2015., 12, 4301-4314
- Tohidi. S., Monajjemi. M.,, and Rustaiyan. A. .,J. Comput. Theor. Nanosci. 2015.,12, 4345-4351
- Zadeh. M. A.A., Lari. H., Kharghanian. L., Balali. E., Khadivi. R., Yahyaei. H., Mollaamin. F., and Monajjemi. M. J. Comput. Theor. Nanosci. 2015.,12, 4358-4367
- Dezfooli. S., Lari. H., Balali. E., Khadivi. R., Farzi. F., Moradiyeh. N., and Monajjemi. M. J. Comput. Theor. Nanosci. 2015.,12, 4478-4488
- Jalilian. H., Sayadian. M., Elsagh. A., Farzi. F., Moradiyeh. N., Soofi. N.S., Khosravi. S., Mohammadian. N. T., and Monajjemi. M. J. Comput. Theor. Nanosci. 2015.,12, 4785-4793
- Farzi. F., Bagheri. S., Rajabzadeh. M., Sayadian. M., Jalilian. H. , Moradiyeh. N., and Monajjemi. M. J. Comput. Theor. Nanosci. 2015.,12, 4862-4872
- Monajjemi. M and Mohammadian. N.T. J. Comput. Theor. Nanosci. 2015.,12, 4895-4914
- Monajjemi, M., Chahkandi, B. Journal of Molecular Structure: THEOCHEM, 2005, 714 (1), 28, 43-60.
- Loiseau. . A. , Willaime. F. , Demoncy. N., Hug. G. and Pascard. H. , Phys. Rev. Lett. 1996.,76 4737,
- K. H. Khoo, M. S. C. Mazzoni and S. G. Louie, Phys. Rev. B 69, 201401R, (2004)
- Bader, R.F.W. Atoms in Molecule: (1990), A quantum Theory (Oxford Univ. press, Oxford).
- Savin , Angew. Chem. Int. Ed.Engl. (1992), 31, 187.
- Jacobsen, H. Can. J. Chem. (2008), 86, 695-702.
- Lu, T.; Chen, F. Acta Chim. Sinica, (2011), 69, 2393-2406.
- Lu, T.; Chen, F J. Mol. Graph. Model, (2012), (38): 314-323.
- Lu, T.; Chen F. J. Comp. Chem. (2012), (33) 580-592.
- Kohn, W.; Sham, LJ. Phys. Rev, 1965, (140) A: 1133-1138.
- Perdew, J .P. Burke, K.; Phys. Rev. Lett.1996, (77): 3865-3868.
- Besler, B.H.; Merz, K.M.; Kollman, P.A. J. comp. Chem, 1990, (11): 431-439, DOI: 10.1002/jcc.540110404
- Chirlian, L.E.; Francl, M.M. J.comp.chem, 1987, (8): 894-905, DOI: 10.1002/jcc.540080616
- Brneman, G.M, Wiberg, K.B. J. Comp Chem, 1990, (11): 361
- Naghsh,F, oriental journal of chemistry, 2015, 31(1). 465-478
- Chitsazan, A, oriental journal of chemistry, 2015, 31(1) 393-408

This work is licensed under a Creative Commons Attribution 4.0 International License.