Application of Magnetic Dicationic Ionic Liquid Phase Transfer Catalyst in Nuclophilic Substitution Reactions of Benzyl Halids in Water
Manouchehr Aghajeri1, Ali Reza Kiasat2 and Bijan Mombeni Goodajdar1*
1Department of Chemistry, Islamic Azad University Omidiyeh Branch, Omidiyeh, Iran
2Department of Chemistry, College of Science, Shahid Chamran University, Ahwaz.
Corresponding Author' s E-mail: bmombini@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/320348
Article Received on : February 24, 2016
Article Accepted on : April 02, 2016
Article Published : 23 May 2016
magnetic dicationic ionic liquid (MDIL) was successfully prepared and evaluated as phase-transfer catalyst for nucleophilic substitution reactions. The reactions was occurred in water and furnished the corresponding benzyl derivatives in high yields. No evidence for the formation of by-product for example benzyl alcohol of the reaction was observed and the products were obtained in pure form without further purification.
KEYWORDS:Green chemistry; Substituted reaction; Phase transfer catalyst
Download this article as:| Copy the following to cite this article: Aghajeri M, Kiasat A. R, Goodajdar B. M. Application of Magnetic Dicationic Ionic Liquid Phase Transfer Catalyst in Nuclophilic Substitution Reactions of Benzyl Halids in Water. Orient J Chem 2016;32(3). |
| Copy the following to cite this URL: Aghajeri M, Kiasat A. R, Goodajdar B. M. Application of Magnetic Dicationic Ionic Liquid Phase Transfer Catalyst in Nuclophilic Substitution Reactions of Benzyl Halids in Water. Orient J Chem 2016;32(3). Available from: http://www.orientjchem.org/?p=16376 |
Introduction
Green chemistry is a major issue of modern chemistry currently. The use of environmentally benign solvent instead of traditional organic solvents is the important and efficient strategy in green chemistry. Water is a promising green solvent for use in chemistry because it is cheap, readily available, and nontoxic. There is increasing recognition that organic reactions carried out in water may offer advantages over those in organic solvents 1-2. However, the poor solubility of reactants in water is the main obstacle to the use of water as reaction solvent.3 One of the most important strategies to overcome this limitation is the utilization of phase transfer catalyst such as ionic liquid (IL).
Phase-Transfer Catalysis (PTC) is a well-known method of promoting reactions between reagents with opposite solubility preferences. In such systems each reactant is dissolved in the appropriate solvent. Commonly, the two solvents are immiscible to one another, and then a phase-transfer catalyst is added to facilitate the transport of one reactant into the other phase. By means of the catalytic step, the enhanced reactivity between the ionic species leads to increase of the rate of the desired reaction 3-6.
As a part of our program aiming at developing selective and environmental friendly methodologies for the preparation of fine chemicals and in continuation of our interest in magnetic ionic liquid promoted organic reactions 7, in this paper, we report on the evaluation of nucleophilic substitution reactions of benzyl halides in the H2O-MDIL system. The method is straightforward and furnish the corresponding benzyl derivatives in high yields.
Results and Discussion
The magnetic dicationic ionic liquid as a catalyst was synthesized according to previously our reported 8. Scheme 1 illustrates the synthetic route for the preparation of MRTDIL synthesized and characterized in this study.
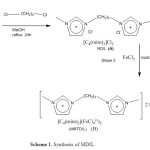 |
Scheme 1: Synthesis of MDIL.
|
To evaluate the catalytic activity of PEG-MDIL, the reaction of benzyl halides with NaN3 was examined as a model reaction. The results are summarized in Table 1.
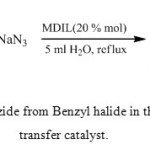 |
Scheme 2: Synthesis of Benzyl Azide from Benzyl halide in the presence of magnetic phase transfer catalyst Click here to View scheme |
Table 1 .Reaction of various benzyl halids with sodium azide in the presence of MDIL in water
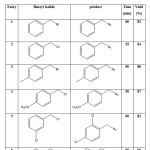 |
Table 1: Reaction of various benzyl halids with sodium azide in the presence of MDIL in water Click here to View table |
We first examined the catalytic ability of MDIL for conversion of benzyl halides to benzyl azide with NaN3 in water at room temperature and under reflux conditions. Circumscribe in reflux condition that results is better. This catalyst acted very efficiently and it converts different benzyl halides to their corresponding benzyl azide in high isolated yields. The obtained results of the reaction are given in Table 1.
1HNMR spectra of the crude products clearly showed the formation of benzyl azide and no evidence for the hydrolysis of benzyl halides to the alcohols was observed, which proved that the reactions proceeded cleanly. On the other hand, in the absence MDIL, the reaction was sluggish and, even after prolonged reaction time, a considerable amount of starting material remained. Moreover, the reaction mixture was contaminated with alcohol. This observation was confirmed with the presence of alcohol on the TLC plate.
The effect of the reaction temperature on the reaction time of benzyl bromide was investigated at reaction temperatures ranging from 25 to 90 °C. The results show that the suitable reaction temperature is 90 °C.
The reaction was carried out in diethyl ether, n-hexane, acetonitrile, dichloromethane, ethyl acetate and water. From the results, it fallows that the best solvent for this reaction is water. Thus, water is an excellent solvent in terms of cost, availability, and environmental impact and shorter reaction times. Benzyl halide bearing activated and deactivated groups were quickly and efficiently converted to the virtually pure corresponding products in high isolated yields. No evidence for the formation of alcohol by products of the reactions was observed and the products were obtained in pure form without further purification.
MDIL showed remarkable reactivity as a Lewis acid reagent and considerably accelerated the reactions. It seems that H2O units in H2O-MDIL encapsulate alkali metal cations, much like crown ethers, and these complexes cause the anion to be activated. The 1-methylimidazol-3-ium units introduced ionic liquid property to the catalyst. In addition, FeCl4– groups at the IL, probably, facilitates the substitution reaction (Scheme 3).
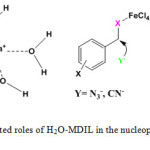 |
Scheme 3: Postulated roles of H2O-MDIL in the nucleophilic substitution
|
Experimental
Material and Methods
Melting points were measured on an Electro thermal 9100 apparatus and are uncorrected.1H & 13CNMR spectra were recorded on a Bruker Advanced DPX 400 MHz instrument spectrometer using TMS as the internal standard in CDCl3. IR spectra were recorded on a BOMEM MB-Series 1988 FT-IR spectrometer. Raman spectroscopy were recorded on a Bruker RFS 100/s Raman spectrometer. Benzyl halides were purchased from Merck Company in high purity. Products were characterized by comparison of their physical and spectroscopic data with those of known samples. .The purity of products and reaction monitoring was accomplished by TLC on silica gel Poly Gram SILG/UV 254 plates.
Typical procedure for the nucleophilic substitution benzyl halides with sodium azide
To a mixture of the benzyl halide (1.0 mmol) and NaN3 (3 mmol) in water (5 ml), MDIL (20 % mol) was added. The suspension was magnetically stirred under reflux conditions for the time shown in Table 1. After complete consumption of starting material as judged by TLC (using n-hexane–ethyl acetate as eluent), the mixture was extracted with ether (2 × 10 ml). The combined organic extracts (dried over CaCl2) were evaporated under reduced pressure. The desired benzyl azide was obtained in good to excellent isolated yields
Conclusion
In conclusion, we have successfully developed a simple and green catalytic procedure for the efficient synthesis of benzyl azides using MDIL and under mild reaction conditions. MDIL can replace the ILs and other homogeneous catalysts with reasonable recovery and reusability and therefore suitable for industrial applications.
Acknowledgments
We gratefully thank Islamic Azad University, Science and Research Branch Omidiyeh for financial support.
References
- Lindström, U. M. Chem. Rev. 2002, 102, 2751-2771.
CrossRef - Siskin, M.; Katritzky, A. R. Chem. Rev. 2001, 101, 825-836
CrossRef - Mahdavi, H.; Tamami, B. React. Funct. Polym. 2005, 64, 179-185.
CrossRef - Xue, S. J.; Ke, S.-Y.; Wei, T.-B.; Duan, L.-P.; and Guo, Y.-L. J. Chin. Chem. Soc. 2004, 51, 1013-1018.
CrossRef - El-Saied, A. A.; Mohamed, A. A.; and El-Gharably, A. A. J. Chin. Chem. Soc. 2004, 51, 983-990.
CrossRef - Kiasat, A. R.; Fallah Mehrjardi, M. J. Chin. Chem. Soc. 2008, 55, 639-642.
CrossRef - (a) Godajdar, B. M.; Kiasat, A. R.; Hashemi, M. M. J. Mol. Liq. 2013, 183, 14-19. (b) B. M. Godajdar, B. M.; A. R. Kiasat, A. R.; M. M. Hashemi, M. M. Heterocycles, 2013, 87, 559-570. (c) Godajdar, B. M.; Kiasat, A. R. J. Chil. Chem. Soc. 2013, 58, 1850-1852. (d) Godajdar, B. M.; Soleimani, S. J. Chin. Chem. Soc. 2014, 61, 447-452
CrossRef - Mombaini Godajadar, B.; Kiasat, A. R.; Ezabadi, A. Orient. J. Chem. 2015, 31, 483-487.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









