The antioxidant activity of ethanol and methanol extracts of sesamemeal by Ultra sonic method in comparison with the synthetic antioxidants in Iranian muttont allow
Mehri Soodbar1, Yousef Ramezan*1 and Parvin Eshratabadi2
1Department of Food Sciences and Technology, Faculty of Advanced Sciences and Technology, Pharmaceutical Sciences Branch, Islamic Azad University, Tehran, Iran
2Institute of Standards and Industrial Research of Iran Karaj, Iran.
Corresponding Author email: y.ramezan@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/320231
Article Received on :
Article Accepted on :
Article Published : 06 Apr 2016
Sesame seeds and its compounds are resistance to oxidative deterioration owing to their natural antioxidants, such as sesamin, sesamolin, sesamol, sesaminol and their glucosides. The purpose of this research is to extract sesame ethanol and methanol antioxidant compounds as by-products by ultrasonic method, then identified by HPLC and has been compared with synthetic antioxidants BHA, BHT, TBHQ on mutton tallow. Ethanolic and methanolic extracts which were extracted by ultrasonic and synthetic antioxidants at concentrations of 0, 50, 100 and 200mg/kg were applied on mutton tallow. The results ofthe oxidative stability based on Rancimat apparatus,Rancimat was operated at 110 °C with an air flow of 18-20 L/h and measures the induction period (IP) of the selected samples,showed that ethanolic and methanolic extracts of sesamemeal at concentrations of 100 and 200mg/kg are not much different from BHT at 50 and 100mg/kg concentrations and also from the methanolic extract at 200 mg/kg concentration in terms of oxidative stability, behave similarly to 50mg/kg concentration of BHA. The results show that the natural antioxidants can be the perfect alternative to synthetic antioxidants.
KEYWORDS:Antioxidants; Mutton Tallow; Oxidative Rancidity; Sesame Meal; Sesamin
Download this article as:| Copy the following to cite this article: Soodbar M, Ramezan Y, Eshratabadi P. The antioxidant activity of ethanol and methanol extracts of sesamemeal by Ultra sonic method in comparison with the synthetic antioxidants in Iranian muttont allow. Orient J Chem 2016;32(2). |
| Copy the following to cite this URL: Soodbar M, Ramezan Y, Eshratabadi P. The antioxidant activity of ethanol and methanol extracts of sesamemeal by Ultra sonic method in comparison with the synthetic antioxidants in Iranian muttont allow. Orient J Chem 2016;32(2). Available from: http://www.orientjchem.org/?p=15202 |
Introduction
The oxidative deterioration of lipids causes rancid odors and flavors as well as the formation of secondary compounds. Products derived from lipid oxidation causes early aging, heart disease, cancer and membrane damage that can be considered as threats to human health. The additional result of antioxidants is an essential way to preserve oxidation of fats and oils in food. Although reports illuminate that synthetic antioxidants that are used as additives inedibleoils can be toxic, and increases manufacturing costs and has hazardous effects on human health and lower efficacy of natural antioxidants. The replacement of synthetic antioxidants by natural antioxidants may have effects due to health implication,therefore demands for usage of natural antioxidants in food system have been enlarged1, 2, 3.
There are many assumptions due to the safety of natural antioxidants so attentions turned to use plant seeds such as sesame regarding to their high content of unsaturated fatty acids, vegetable protein, soluble fiber, flavonoids and other compounds,which have antioxidant activity4.
Sesame seed (Sesamumindicum) is recognized as a source of high activity antioxidant with to copherol and lignans as primary compounds. Sesame is one of the most edible oil seeds which had been used through East Africa where it is generally grown for grain and oil. The chemical composition of sesame indicated that the seed is a significant source of oil (44 – 58%), protein (18 – 25%), carbohydrate (~13.5%) and ash (~5%) 5, 6.
Due to the sesame seed’s considerable amount of nutrients, including protein, essential fatty acids, vitamin E, minerals,and lignans(including sesamin, sesamolin, and sesaminol) has special significance in terms of nutrition and low production cost. Its lignans may have antioxidants and anti-inflammatory properties4, 7.
Sesame seed oil significantly shows high resistance against autoxidation and also avoids the oxidation of linoleicacid. These antioxidants have nocolor, taste and odoratlow concentrations in comparison with synthetic antioxidants such as BHA and BHT in small amounts8, 9.
Sesame seednaturally containslignans and lignan-glucosides as the functional components which are mainly related to its antioxidant’s properties. The major lignans in sesame seed are sesamin and sesamolinthat are progressively improved to two phenolic antioxidants, sesaminol and sesamol9, 10.
Sesamin and sesamolin compounds are higher in oil solublelignan compounds in compare with other lignan values. Sesamin makes up more than half of the lignan contained compounds. These compounds function in controlling lipid metabolism, lowering cholesterol, enhancing hepatic fatty acid oxidation enzymes,protecting patients against brain disorders and have medicinal properties. They are also anticancer and antioxidant11, 12, 13.
In this study,sesame seeds’mealas by-product of the oil industry,is used for the extraction of sesame seeds’ antioxidants. In these circumstances, phenolic compounds, radical inhibitory activity, power reduction’s effect and antioxidant activity of sesamemeal are significantly increased14, 15.
The research made an effort to use sesame meal for extracting natural antioxidants and use its ability to compare antioxidants activity with synthetic antioxidants.
Materials and Methods
Sesamemeal samples which were used in this study were provided from a storeina local market that is working in a line with sesame oil production. Also Iranian mutton tallow that is aimed in the study was consumed in a local market in Tehran. Defatted sesame meal was powdered by mortar and then it was used in next steps of extractions. The standard of sesamol was obtained from Sigma Chemical Co. (St. Louis, MO, USA), and all solvents were of HPLC or analytical grade (Merck).
Standards Preparation
Stock solutions of sesame compounds (2 μg/mL) were prepared in methanol, which was stable for weeks in the dark and at 0°C. The stocks were used for the preparation of working standards (0.05, 0.1, 0.2, 0.3, 0.4, 0.5 μg/mL) and calibration curve.
Extraction by Ultrasonic Bath
400 ml of ethanol and methanol was poured throw 300 g of sesame meal powder predicated in an ultrasonic bath with room temperature for 45 minutes, degassing operations were done at the frequency of 60 Hz. Degassing process of extracting, facilitated the extraction of phenolic compounds during the ultrasound.
Identification of Extracted Compounds By HPLC
The method was based on direct extraction of polar biophenol compounds of sesame meal by using solutions of ethanol and methanol that had been detected by HPLC with 280 nm UV.Three solvents were used for the washing gradient, such as, water containing 0.2% phosphoric acid (V/V), methanol and acetonitrile. Washing solutions had to be degassed 6.
Incorporation of antioxidant compounds with Iranian mutton tallow to evaluate oxidative stability
The synthetic antioxidants but ylated hydroxyanisole (BHA), butylatedhydroxytoluene (BHT) and Tertiary butylhydroquinone(TBHQ),ethanolic and methanolic extracts were applied on the mutton tallow that obtained by ultrasonic extraction methods at concentrations of 0, 50, 100 and 200 mg/kg, until evaluation was started.
Rancimat Analysis
The induction period of the samples were evaluated by MetrohmRancimat apparatus model 743(Herisau / Switzerland). The instrument was operated at 110 °Cwith an air flow rate of 18-20 L/h. The induction period was recognized by a sharp change in the slope on the chart. A tangent was drawn from the slope to intersect an extension of the baseline and the distance of this intersection was a measure of the induction time from the start17.
Statistical Analysis
The data were analyzed by using a complete randomized design. To determine significant data, one-way experiments (ANOVA) were used. The Tukey’s test was applied to compare means at 5 percent by using Minitb 16 software. All results are the consequences of ± standard deviation and were performed for at least 3 times.
Results and Discussions
The results of HPLC identification of antioxidant extracts from ethanolic and methanolic sesame meal extract by ultrasonic bath extraction method are shown in Fig.1 and Fig.2. It is observed that ethanolic and methanolic sesame meal antioxidant extracts by ultrasonic bath extraction method were evaluated and the natural antioxidants vizsesamol, sesaminol T- glucoside, sesaminol D-glucoside, sesamin and sesamolin were identified respectively.
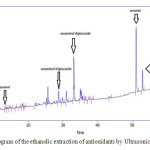 |
Figure 1: chromatogram of the ethanolic extraction of antioxidants by Ultrasonic extraction method
|
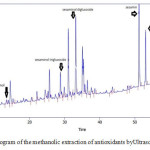 |
|
Iranian muttont allow oxidativestability evaluation was conducted by Rancimat apparatus. Table 1 are presented oxidative stability of Iranian muttont allow incorporation with synthetic antioxidants, ethanolic and methanolic antioxidant extracts obtained from the ultrasonic bath extraction method which were added into mutton tallow, at concentrations of 0, 50, 100 and 200 mg/kg.
Table 1: Natural And Synthetic Antioxidant Activity Obtained By RancimatApparatus (H)
|
Sample (mg/kg) |
Induction Period (h) |
|
mutton tallow oil |
4.25±0.247m |
|
mutton tallow oil + 50 mg/kg BHA |
12.1±0.863h |
|
mutton tallow oil + 100 mg/kg BHA |
18.47±0.092g |
|
mutton tallow oil +200 mg/kg BHA |
19.04±0.304fg |
|
mutton tallow oil + 50 mg/kg BHT |
7.93±0.042jkl |
|
mutton tallow oil + 100 mg/kg BHT |
9.35±0.007ijk |
|
mutton tallow oil +200 mg/kg BHT |
11.43±1.011hi |
|
mutton tallow oil + 50 mg/kg TBHQ |
20.94±1.633f |
|
mutton tallow oil + 100 mg/kg TBHQ |
29.39±0.849e |
|
mutton tallow oil + 100 mg/kg TBHQ |
34.79±0.643d |
|
mutton tallow oil + 50 mg/kg ethanolic ultrasonic extract |
6.75±0.325l |
|
mutton tallow oil + 100 mg/kg ethanolic ultrasonic extract |
7.28±0. 78kl |
|
mutton tallow oil + 200 mg/kg ethanolic ultrasonic extract |
7.42±0.106kl |
|
mutton tallow oil + 50 mg/kg methanolic ultrasonic extract |
6.83±0.445l |
|
mutton tallow oil + 100 mg/kg methanolic ultrasonic extract |
7.47±0.12jkl |
|
mutton tallow oil + 200 mg/kg methanolic ultrasonic extract |
9.85±0.12hij |
Discussions
Most researchers have been attributed notable oxidative stability of sesame seed oil to non-saponification substances to date. In this study, oxidativest ability of Iranian mutton tallow with ethanolic and methanolic antioxidant extracts obtained by ultrasonic bath extraction techniques were compared with synthetic antioxidants at concentrations of 0, 50, 100 and 200 mg/kg at 110º C by Rancimat apparatus.
Sujaet al.(2004)stated that applying sesamemeal extractat 5, 10, 50 and 100 mg/kg concentrations in soybean, sunflower and safflower oil during storage,increased oils’ oxidative stability. The obtained antioxidant extracts, had a higher impact on oxidative stability than BHT at 200 mg/kg18.
Mohdalyet al. (2011)and Abdelazimet al.(2013)reported an assessment between the antioxidant activity of the sesamemeal extracts and synthetic antioxidants on vegetable oils. So there were similarities between the results of this study and their studies about antioxidant activity of the sesamemeal extracts and synthetic antioxidant son the mutton tallow and on vegetable oils’ oxidative stability 19, 20.
In the present work, there is no significant difference(P>0.05)between oxidative stability of mutton tallow at 50 and 100mg/kg ofBHT and 100 mg/kg of ethanolic and methanolic antioxidant extracts gained from the ultrasonic bath method.
Oxidative stability significantly lowers the induction time(P<0.05),at 50 mg/kg ethanolic and methanolic extracts obtained by ultrasonic bath extraction technique, in comparison with BHT at 100mg/kg concentration. However,no significant difference weas observed at concentration of 50mg/kg of ethanolic and methanolic antioxidant substances extracted by ultrasonic bath method and BHT (P>0.05).
It was observed that the methanolic extract obtained by ultrasonic bath extraction method at concentration of 200mg/kg,behaved like 50mg/kg concentration of BHA. Also there were no significant differences between the concentrations of 100mg/kg methanolic extract with other concentrations of methanolic extract. TBHQ is the most efficient antioxidant followed by BHA, BHT, methanolic and ethanolic extracts, in decreasing order.
Conclusion
According to the results obtained in this study, it can be stated that the senatural antioxidants have the ability to substitute the synthetic antioxidants or whether synthetica ntioxidants can be used with natural antioxidant to increase the stability of lipid rancidity against autoxidation process in food industry.
Acknowledgment
The authors are grateful to Institute of National Iranian Standards and Industrial Research for their technical assistance with sample extractions, HPLC and Rancimat analysis in this study.
References
- Fahimi,R.; Elhamirad, A.H.; Hadad Khodaparast, M.H.; Estiri, S.H.; Armin,M.; Askari,B.; Mokhtari, F.; Ghasemi, S. National Food Science Congress. 2011.
- 2. Konsoula, Z.; Liakopoulou-Kyriakides, M. LWT-Food Sci. Technol. 2010, 43, 1379-1386.
- Moure, A.; M.Cruz, J.; Franco, D.; Dominguez, J.M.; Sineiro, J.; Dominguez, H.; Nunez, M J.; Parajo, J.C. Food Chem. 2001, 72, 145-171.
CrossRef - Ghani, N. A.; Shawkat, M. S.; Umran, M. A. Current Res. J. Biol. Sci. 2012, 4, 159-163.
- Fitrotin, U.; Utami, T.; Hastuti, P.; Santoso, U. Int. J. Biol. Sci. 2015, 4, 56-61.
- Rizki, H.; Kzaiber, F.; Nablousi, A.; Elharfi, M.; Ennahli, S.; Hanine, H. Int. j. adv. res. sci. eng. technol.2015, 2, 392-397.
- Khadem Haghighi, M.; Alipoor, B.; Malek Mahdavi, A.; Eftekhar Sadat, B.; Jafarabadi, M.; Moghadam, A. 2014. Acta med. Iran. 2014, 53, 207-213.
- Suja, K.P.;Jayalekshmy, A.; Arumughan, C. Food Chem. 2005,91, 213–219.
CrossRef - Ramezan, Y.; Ghavami, M.; Bahmaei, M.; Givianrad, M. H.;Hemmasi, A. H. (2015). Orient. J. Chem. 2015,31(3), 1389–1394.
CrossRef - Chakraborthy, G.S.; Sharma, G.; Kaushik, K. N. J. Herb. Med. Toxic.2008,2, 15-19.
- Rangkadilok, N.; Pholphana, N.; Mahidol,C.; Wongyai, W.; Saengsooksree, K.; Nookabkaew, S.; Satayavivad, J. Food Chem. 2010, 122, 724–730.
CrossRef - Shirato-Yasumoto, S.; Komeichi, M.; Okuyama, Y.; Horigane, A. Sabrao J. Breed. Genet. 2003, 35, 27-34.
- Zhou, J-C.; Feng, D-W.; Zheng,G-S. J. Food Eng. 2010, 100, 289–293.
CrossRef - Hassan, M.A. 2013. World J. Dairy Food Sci. 2013, 8, 51-57.
- Elleuch, M.; Besbes , S.; Roiseux, O.; Blecker, C.; Attia, H. Food Chem. 2007,103, 641-650.
CrossRef - COI/T.20/Doc No 29. 2009. Determination of biophenols in olive oils by HPLC.
- ISO6886. 2006. Animal and vegetable fat and oil- determination of oxidative stability (accelerated oxidation method).
- Suja, K.P.; Abraham, J.T; Thamizh, S.N, Jayalekshmy, A.; Arumughan, C. Food Chem. 2004, 84, 393–400.
CrossRef - Abdelazim, A.; Mahmoud, A.; Ramadan-Hassanien, M.F. Food Sci. Techno.2013,50, 868–878.
- Mohdaly, A.A.; Smetanskaa, I.; Ramadan, M.F; Sarhan, M.A.; Mahmoud., A. Ind. Crop. Prod. 2011, 34, 952– 959.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









