Synthesis of C–4–Allyloxy–3–Methoxyphenylcalix[4]resorcinarene from Vanillin and Its Application as Adsorbent of Pb(II) Metal Cation
Endhy Putra Kesuma1, Jumina1, Keisuke Ohto2 and Dwi Siswanta1,*
1Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Gadjah Mada, Yogyakarta 55281, Indonesia
2Department of Chemistry and Applied Chemistry, Faculty of Science and Engineering, Saga University, 1–Honjo, Saga 840–8502, Japan.
Corresponding author Email: dsiswanta@ugm.ac.id
DOI : http://dx.doi.org/10.13005/ojc/320202
Article Received on :
Article Accepted on :
Article Published : 05 May 2016
C–4–allyloxy–3–methoxyphenylcalix[4] resorcinarene had been synthesized from vanillin and its application as adsorbent for Pb(II) metal ion had been carried out. The synthesis was conducted in three steps to obtain the product as pink solid in 78.17% yield. The structure elucidation of the product was performed by IR, 1H–NMR and 13C–NMR. Adsorption experiments were carried out in a batch system under variation of medium acidity, interaction time and initial metal concentration. Adsorption kinetics was studied using Lagergren and Ho model, while adsorption isotherm was analyzed by Langmuir and Freundlich equations. The result showed that the Pb(II) adsorption was optimum at pH 4 and at interaction time of 30 minutes. The kinetic study showed that the adsorption of Pb(II) followed pseudo–second order of Ho model with adsorption rate (k) of 1.176 g mg–1 minute–1. The adsorption followed the Langmuir isotherm model with equilibrium constant (K) was 7.28×10–5 L mol–1, adsorption capacity (qm) was 1.538 mmol g–1 (318.67 mg/g) and adsorption energy was 33.67 kJ mol–1.
KEYWORDS:calix[4]resorcinarene; vanillin; adsorption; Pb(II)
Download this article as:| Copy the following to cite this article: Kesuma E. P, Jumina, Ohto K, Siswanta D. Synthesis of C–4–Allyloxy–3–Methoxyphenylcalix[4]resorcinarene from Vanillin and Its Application as Adsorbent of Pb(II) Metal Cation. Orient J Chem 2016;32(2) |
| Copy the following to cite this URL: Kesuma E. P, Jumina, Ohto K, Siswanta D. Synthesis of C–4–Allyloxy–3–Methoxyphenylcalix[4]resorcinarene from Vanillin and Its Application as Adsorbent of Pb(II) Metal Cation. Orient J Chem 2016;32(2). Available from: http://www.orientjchem.org/?p=15768 |
Introduction
Global industrial advance gives a positive effect to escalate the economy level, which triggers advance in another sector, but also give negative effect such as pollutants and waste, which contains toxic materials. Various water pollutants such as organic compounds, inorganic, as well as heavy metal waste had been identified. One of the hazardous heavy metals was lead (Pb) due to the toxic effects of metals in humans and the environment. Lead compounds are found in the form of Pb(II) and some in the form of Pb (IV). Lead is widely used as industrial raw materials, such as battery, paint and dye industries1. Acute lead poisoning can cause physiological disorders and chronic toxic effects on children may experience disturbance on the physical and mental growth 2,3. Therefore, it is necessary to treat waste containing Pb(II).
Many methods have been developed to process the contaminated waste into an acceptable limit quality of waste water, such as coagulation 4,5, electrodialysis 6–8, and adsorption9,10. Adsorption has been shown to be an economically practicable alternative method for lead removal from wastewater 11. Several adsorbents have been developed are chitosan 10,12,13, zeolite 14–16, activated carbon 17–19, and calixarene 20–24.
Calixarene is a supramolecular cyclic compound with phenolic groups connected by methylene bridge. A derivative of calix[n]resorcinarene is produced by reacting resorcinol and an aldehyde compound in acid condition25. Product can be modified in its narrow rim, wide rim, meta position, bridging methylene, or outer face 24,26–28. Vanillin is an aldehyde compound with aromatic rings and methoxy group, which can react with resorcinol, producing calix[4]resorcinarene derivative which is water soluble. A phenolic group in vanilin is a good leaving group, which is possible to be substituted or modified. Calix[4]resorcinarene has been applied as adsorbent for heavy metals 20,21,23,24,29. In this work, in order to improve the adsorption capacity, calix[4]resorcinarene with allyloxy groups were synthesized and examined its adsorption capability for Pb(II) ion in aqueous solution.
Experimental Section
Materials
Materials used in this research are fresh Bromic Acid 47%, allyl alcohol, vanillin, resorcinol, and other necessary reagents, all are p.a. grade from Merck. Stock solutions of the Pb(II) ions were prepared by dissolving Pb(NO3)2 (Merck) in demineralized double distilled water. Different concentrations of test solutions of Pb(II) were prepared by proper dilution of the stock solutions. The pH of the solution was adjusted by the addition of 0.1 M HCl (Merck) and 0.1 M NaOH (Merck).
Instrumentation
Characterization of the compounds was accomplished using FTIR Shimadzu–Prestige 21, NMR Agilent VNMR 400MHz, GC–MS Shimadzu QP–2010S. AAS Perkin Elmer 3110 was used to measured the Pb(II) ion concentration in the aqueous solution.
Procedure
Synthesis of C–4–allyloxy–3–methoxy phenylcalix[4]resorcinarene
The synthesis of C–4–allyloxy–3–methoxyphenylcalix[4]resorcinarene was carried out in 3 steps, synthesis of 3–bromo–1–propene, synthesis of 4–allyloxy–3–methoxy–benzaldehyde, and synthesis of calixarene derivative.
Synthesis of 3–bromo–1–propene
Bromic acid 47% reacted with 8.5 mL allyl alcohol 99% using sulfuric acid catalyst in 3–neck flask, then stirred for 2 hours and distilled at 70 °C. The product was characterized using FTIR and GC–MS.
Synthesis of 4–allyloxy–3–methoxy benzaldehyde
Vanillin was dissolved in acetone, then reacted with allyl bromide and anhydrous potassium carbonate in 3–neck flask, refluxed for 8 hours, cooled down and then the solvent was evaporated. Solid residue was dispersed in aquadest and extracted using ether, then washed using 2 M sodium hydroxide. The solvent was evaporated, and the solid residue was characterized using FTIR and GC–MS.
Synthesis of calixarene derivative. Allyl vanillin was dissolved in ethanol and reacted with resorcinol and sulfuric acid catalyst in 3–neck flask, refluxed for 24 hours, then cooled down. The solid was vacuum filtrated using Buchner and the washed with ethanol:aquadest 1:1 until neutral. The residue was dried in oven, then characterized using FTIR, 1H–NMR and 13C–NMR.
Adsorption of Pb solution
Adsorption experiments were carried out in a batch sistem under variation of medium acidity, interaction time and initial metal concentration. Adsorption kinetics was studied using Lagergren and Ho equations, while adsorption isotherm was analyzed using Langmuir and Freundlich model equations.
Effect of pH
Pb(II) solution 10 mL (10 ppm) was contacted with the 0.01 g calixarene product, with pH variation on 3, 4, 5, 6, then stirred for 3 h. The filtrate that was containing remaining Pb(II) was analyzed using AAS.
Effect of adsorption time
Pb(II) solution 10 mL (10 ppm) was contacted with the 0.01 g calixarene product, at optimum pH, then stirred for variation of contact time of 3, 5, 15, 30, 60, 120 min. Filtrate containing remaining Pb(II) was analyzed using AAS.
Effect of adsorbate concentration
Pb(II) solution (concentration variation of 10, 20, 50, 100 ppm) 10 mL was contacted with the 0.01 g calixarene product, at optimum pH and contact time. Filtrate containing remaining Pb(II) was analyzed using AAS.
Results and Discussion
Synthesis of C–4–allyloxy–3–methoxy phenylcalix[4]resorcinarene
![Figure 1. Synthesis of C–4–allyloxy–3–methoxyphenylcalix[4]resorcinarene](http://www.orientjchem.org/wp-content/uploads/2016/05/Vol32_No2_Synt_End_Fig1-150x150.jpg) |
Figure 1: Synthesis of C–4–allyloxy 3–methoxyphenylcalix[4]resorcinarene Click here to View figure |
Synthesis of 3–bromo–1–propene
The product was 1.21 g colorless liquid with pungent odor and boiling point of 70 °C. Characterization using FTIR showed C=C absorption at 1635 cm–1, strong absorption at 987 and 925 cm–1 which were C=CH2 terminal, and C–Br at 686 cm–1. GC–MS characterization showed a peak at 2.718 minute retention time, with a parent peak at m/z 120 and base peak at m/z 41.
Synthesis of 4–allyloxy–3–methoxybenzaldehyde
The product was 1.48 g brownish yellow liquid with characteristic odor, boiling point of 78 °C. Characterization using FTIR showed C=O carbonyl absorption at 1689 cm–1, absorption at 910 and 990 cm–1 were C=CH2 terminal, and disappearance of O–H absorption at 3200 cm–1. GC–MS characterization showed a single peak at 20.655 minute retention time, with a parent peak at m/z 192 and base peak at m/z 41.
Synthesis of calixarene derivative
The product was 1.11g pink odorless solid with melting point of 238 °C. Characterization using FTIR shows C–O–C ether absorption at 1257 and 1219 cm–1, C=CH2 terminal at 925 and 1026 cm–1, reappearance of OH vibration at 3411 cm–1 and disappearance of C=O and C–H aldehyde absorption at 1689 cm–1, 2854 and 2731 cm–1 respectively. 1H–NMR characterization exhibited 12 types of proton, singlet CH3 methoxy group was at δ = 3,40 ppm, allyloxy group was at δ = 4.44, 5.21, 5.35, 6.00 ppm. Methylene bridge was singlet peak at δ =5.59 ppm, while vanillin aromatic ring showed peaks at δ = 6.14, 6.23, and 6.41 ppm. Resorcinol aromatic ring were at δ = 6.08 and 6.35 ppm, and its OH singlet peak was at δ = 8.48 ppm. Characterization using 13C–NMR showed 15 types of carbon. Methylene bridge carbon was at δ = 40.38 ppm, and methoxy carbon was at δ = 55.51 ppm. Allyoxy group was at δ = 69.51, 113.45, 139,30 ppm. Vanillin carbon was at δ = 112.68, 117.06, 120.95, 137.45, 145.44, and 148.34 ppm. Resorcinol carbon was at 102.43, 122.25, 135.49, 152.66 ppm
Adsorption of Pb solution
Effect of pH
The effect of the solution acidity on the Pb(II) adsorption was examined by equilibrating under stirring the Pb(II) nitrate solution adjusted at pH 3 to 7 with the adsorbent and measured the remainder of Pb(II) present in the solution that was not adsorbed. Pb2+ species present in maximum amount at pH 1–6 and above pH 6 it begins to decline. Therefore, the adsorption was done on the variation of the pH in the range 3–6 to determine the amount of Pb2+ in water, taking into account that pH range, Pb2+ species are the maximum amount and the adsorbent is not in a state of protonated, so hopefully there will be conditions of adsorption achieve optimum. Adsorption time was selected at 3 h. After adsorption equilibrium achieved, the solution was filtered and then analyzed using AAS. The reference solution was also treated similarly, but without the addition of the adsorbent. The effect of the acidity of the adsorbent C–4–allyloxy–3–methoxyphenylcalix[4] resorcinarene presented in Fig. 2. The optimum pH was observed at pH 4, as expected that at lower pH there would be high concentration of H+ that competing with Pb2+ ion to bind with the adsorbent.
Effect of adsorption time
The effect of contact time between the adsorbent and the metal cation is observed by the interaction between the adsorbent C–4–allyloxy–3–methoxyphenylcalix[4] resorcinarene and metal cations Pb (II) at pH optimum with a variety of contact time by means of stirring using a magnetic stirrer. The effect of the contact time of the adsorbent was presented in Fig. 3. The result shows that the contact time determines the amount of metal ions adsorbed (in mmol g–1). The longer the interaction time, the amount of adsorbed metal increased, reached maximum at 30 min, and became constant after 30 minutes. Pb(II) adsorption reached its optimum at 30 min with an adsorption capacity of 0.0505 mmol g–1 (10.46 mg g–1). Rapid adsorption at early time due to the availability of active groups on the adsorbent C–4–allyloxy–3–methoxyphenylcalix[4] resorcinarene. When it reached the optimum time, there was the condition of adsorption equilibrium when all active sites have been occupied by metal cations. Similar trends were also reported for the adsorption of Pb(II) onto other adsorbents such as silica–gel supported hyperbranched polyamidoamine dendrimers30, chitosan–polyacrylonitrile blend31, C–methylcalix[4]resorcinarene24.
![Figure 2 Effect of pH on the Pb(II) adsorption by C–4–allyloxy–3–methoxyphenylcalix[4] resorcinarene (Co =10 mg L−1, and adsorbent dose: 1 g L–1)](http://www.orientjchem.org/wp-content/uploads/2016/05/Vol32_No2_Synt_End_Fig2-150x150.jpg) |
Figure 2: Effect of pH on the Pb(II) adsorption by C–4–allyloxy–3–methoxyphenylcalix[4] resorcinarene (Co =10 mg L−1, and adsorbent dose: 1 g L–1) Click here to View figure |
The adsorption kinetics of C–4–allyloxy–3–methoxyphenylcalix[4] resorcinarene for Pb(II) at room temperature is shown in Fig. 3. Adsorption kinetics are crucial in the adsorption study as it provides important understandings into the adsorption mechanism. Fig 3 shows that adsorption equilibrium was reached at 30 min. In order to ensure complete adsorption, the contact time was fixed at 60 min in the following experiments. Adsorption kinetic parameters, such as the rate constants and equilibrium adsorption capacities, which can provide valuable insights into wastewater treatment process design, are of great importance for the application of adsorbents. In order to evaluate the kinetic adsorption mechanism, pseudo–first order and pseudo– second order models, as shown in Eqs. (2) and (3), were employed to interpret the experimental data [4,33,38].
ln(qe − qt) = ln qe − k1t
qt = k2q2 + qe te (2)
where qe is the amount of metal adsorbed at equilibrium (mmol g−1), qt is the amount of metal ions adsorbed at any time (mmol g−1), k1 and k2 are the rate constant of pseudo–first order (min−1) and pseudo–second order (g mmol−1 min−1) adsorption, respectively. The plots of log(qe− qt) versus t and t/q versus t were employed to test pseudo–first–order and pseudo–second–order models, and the fitting results are shown in Fig. 4. Pseudo–second order model of Ho provides better correlation coefficients than pseudo–first order model (Lagergren), suggesting that pseudo– second order model is more suitable to describe the adsorption kinetics processes. Also, the equilibrium adsorption capacities calculated (qe,cal) based on the pseudo–second order model are much better in agreement with the experimental data (qe,exp), further demonstrated that pseudo–second–order model is more suitable to describe the kinetic adsorption processes. Pseudo second order Ho is more linier with R2= 0.999 and equation y = 0.097x + 0.008, k1 = 1.176 g mg–1 minute–1.
![Figure 3 Adsorption kinetics of C–4–allyloxy–3–methoxyphenylcalix[4] resorcinarene for Pb(II) (adsorption conditions: 10 mL Pb(II) 10 mg L–1, 10 mg adsorbent, pH 4.0).](http://www.orientjchem.org/wp-content/uploads/2016/05/Vol32_No2_Synt_End_Fig3-150x150.jpg) |
Figure 3: Adsorption kinetics of C–4–allyloxy–3–methoxyphenylcalix[4] resorcinarene for Pb(II) (adsorption conditions: 10 mL Pb(II) 10 mg L–1, 10 mg adsorbent, pH 4.0). Click here to View figure |
Effect of adsorbate concentration
Adsorption increases as adsorbate concentration rises, with an optimum adsorbate concentration discovered at 50 ppm, because active sites in adsorbent has reached equilibrium. Then adsorption isotherm was analyzed using Langmuir and Freundlich equations. The Langmuir isotherm model was observed to be more liniar with R2 = 0.982 and equation y = 6555x + 0.999. Langmuir isotherm model is based on monolayer adsorption and all active sites are homogeneous. KL was obtained as 7.283 x 105 L mol–1, Xm was 0.1538 mmol g–1 (31.87 mg g–1), and adsorption energy was 33.67 kJ mol–1. Adsorption mechanism followed hydrogen bond formation between active sites of the adsorbent and the solvated Pb2+ ion.
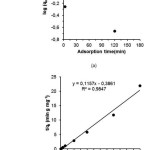 |
Figure 4: Adsorption kinetic model: (a) Lagergren and (b) Ho Click here to View figure |
![Figure 5. (a) Adsorption of C–4–allyloxy–3–methoxyphenylcalix[4] resorcinarene for Pb(II) (adsorption conditions: 10 mL Pb(II), 10 mg adsorbent, pH 4.0 and contact time 60 min). (b) Langmuir and (c) Freundlich adsorption isotherm model.](http://www.orientjchem.org/wp-content/uploads/2016/05/Vol32_No2_Synt_End_Fig51-150x150.jpg) |
Figure 5: (a) Adsorption of C–4–allyloxy–3–methoxyphenylcalix[4] resorcinarene for Pb(II) (adsorption conditions: 10 mL Pb(II), 10 mg adsorbent, pH 4.0 and contact time 60 min). (b) Langmuir and (c) Freundlich adsorption isotherm model. Click here to View figure |
Conclusion
C–4–allyloxy–3–methoxyphenyl calix[4]resorcinarene from vanillin could be synthesized in three steps, from vanillin with 3–bromo–1–propene then resorcinol to produce C–4–allyloxy–3–methoxyphenylcalix [4]resorcinarene. This product was obtained as pink solid in 78.17% yield having melting point of more than 200 °C. The structure elucidation of the product was performed by IR, 1H–NMR and 13C–NMR.
The result adsorption experiments showed that the amount of adsorbed metal increased with the increase of pH and interaction time until finally reached the optimum condition at pH 4 and at interaction time 30 minutes. The kinetic study showed that the adsorption of Pb(II) followed pseudo–second order of Ho with adsorption rate (k) of 1.176 g mg–1 minute–1. The isotherm study showed that the adsorption followed the Langmuir isotherm model. The calculated equilibrium constant (KL) was 7.283×10–5 L mol–1, maximum adsorption capacity (Xm) was 1.538 mmol g–1 and adsorption energy was 33.67 kJ mol–1.
Acknowledgement
The authors thank to the Directorate of research and Community Service (DP2M–DIKTI), the Ministry of Education and Culture, Republic of Indonesia, who has provided financial support for the implementation of this research through International Collaboration and International Publication Grant budget year 2014–2015.
References
- Yurtsever, M.; Şengil, İ. J. Hazard. Mater. 2009, 163, 58–64.
CrossRef - Singh, C. K.; Sahu, J. N.; Mahalik, K. K.; Mohanty, C. R.; Mohan, B. R.; Meikap, B. C. J. Hazard. Mater. 2008, 153 (1-2), 221–228.
CrossRef - Acharya, J.; Sahu, J. N.; Mohanty, C. R.; Meikap, B. C. Chem. Eng. J. 2009, 149 (1-3), 249–262.
CrossRef - Wang, S.; Ang, H.; Tadé, M. Chemosphere 2008, 72 (11), 1621–1635.
CrossRef - Jiang, J.; Lloyd, B. Water Res. 2002, 36 (6), 1397–1408.
CrossRef - Mohammadi, T.; Razmi, A.; Sadrzadeh, M. Desalination 2004, 167, 379–385.
CrossRef - Mohammadi, T.; Moheb, A. Sep. Purif. Technol. 2005, 41 (1), 73–82.
CrossRef - Sadrzadeh, M.; Mohammadi, T. Chem. Eng. J. 2008, 144 (3), 431–441.
CrossRef - Asgari, M. S.; Zonouzi, A.; Rahimi, R.; Rabbani, M. Orient. J. Chem. 2015, 31 (3), 1537–1544.
CrossRef - Moganavally, P.; Deepa, M.; Sudha, P. N.; Suresh, R. Orient. J. Chem. 2016, 32 (1), 441–453.
CrossRef - Peternele, W. S.; Winkler-Hechenleitner, A. a.; Gómez Pineda, E. a. Bioresour. Technol. 1999, 68 (1), 95–100.
CrossRef - Sun, S.; Wang, A. React. Funct. Polym. 2006, 66 (8), 819–826.
CrossRef - Srinivasan, S.; Chelliah, P.; Srinivasan, V. Orient. J. Chem. 2016, 32 (1), 671–680.
CrossRef - Wang, X.; Shao, D.; Hou, G.; Wang, X.; Alsaedi, A.; Ahmad, B. J. Mol. Liq. 2015, 207, 338–342.
CrossRef - EL-Mekkawi, D. M.; Selim, M. M. J. Environ. Chem. Eng. 2014, 2 (1), 723–730.
- Nguyen, T. C.; Loganathan, P.; Nguyen, T. V.; Vigneswaran, S.; Kandasamy, J.; Naidu, R. Chem. Eng. J. 2015, 270, 393–404.
CrossRef - Mohamad Nor, N.; Lau, L. C.; Lee, K. T.; Mohamed, A. R. J. Environ. Chem. Eng. 2013, 1 (4), 658–666.
CrossRef - Abbas, M.; Kaddour, S.; Trari, M. J. Ind. Eng. Chem. 2014, 20 (3), 745–751.
CrossRef - Awwad, N. S.; El-Zahhar, A. A.; Fouda, A. M.; Ibrahium, H. A. J. Environ. Chem. Eng. 2013, 1 (3), 416–423.
CrossRef - Handayani, D. S.; Siswanta, D.; Ohto, K.; Kawakita, H. Indones. J. Chem. 2011, 11 (2), 191–195.
- Adhikari, B. B.; Kanemitsu, M.; Kawakita, H.; Ohto, K. Chem. Eng. J. 2011, 172 (1), 341–353.
CrossRef - Adhikari, B. B.; Gurung, M.; Chetry, A. B.; Kawakita, H.; Ohto, K. RSC Adv. 2013, 3 (48), 25950–25959.
CrossRef - Matsumiya, H.; Masai, H.; Terazono, Y.; Iki, N.; Miyano, S. Bull. Chem. Soc. Jpn. 2003, 76 (1), 133–136.
CrossRef - Jumina, J.; Sarjono, R. E.; Siswanta, D.; Santosa, S. J.; Ohto, K. J. Korean Chem. Soc. 2011, 55 (3), 454–462.
CrossRef - Chen, Y. Synthesis of octa-tailed calix resorcinarenes and characterization of Langmuir-Blodgett films on these compounds., University of Windsor, 1999.
- Jumina; Ratnaningsih Eko Sarjono, Brajna Paramitha, Ika Hendaryani, D. S.; Sri Juari Santosa, Chairil Anwar, Hardjono Sastrohamidjojo, K. O. and T. O. J. Chinese Chem. Soc. 2007, 54, 1167–1178.
- Falana, O.; Al-Farhan, E.; Keehn, P.; Stevenson, R. Tetrahedron Lett. 1994, 35 (1), 65–68.
CrossRef - Timmerman, P.; Verboom, W.; Reinhoudt, D. Tetrahedron 1996, 52 (8), 2663–2704.
CrossRef - Solangi, I. B.; Memon, S.; Bhanger, M. I. Anal. Chim. Acta 2009, 638 (2), 146–153.
CrossRef - Niu, Y.; Qu, R.; Sun, C.; Wang, C.; Chen, H.; Ji, C.; Zhang, Y.; Shao, X.; Bu, F. J. Hazard. Mater. 2013, 244-245, 276–286.
CrossRef - Anitha, T.; Kumar, P. S.; Kumar, K. S.; Ramkumar, B.; Ramalingam, S. Process Saf. Environ. Prot. 2015, 98, 187–197.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









