Nonaqueous preparation of stable silver nano particles dispersions from organic sulfonic acids.
Valentina Glushko*, Lidiya Blokhina, Natalya Sadovskaya and Vadim Kozhukhov
Federal State Unitary Enterprise «State Scientific Research Institute of Chemical Reagents and High Purity Chemical Substances» (FSUE «IREA»), 3, Bogorodskiy val, Moscow, 107076, Russia. Corresponding author email: tetrazoli@yandex.ru
DOI : http://dx.doi.org/10.13005/ojc/320204
Article Received on :
Article Accepted on :
Article Published : 23 Apr 2016
The conditions for stable silver nano particles dispersions synthesis from organic sulfonic acids in an anhydrous medium of ethylene glycol and its methyl ester were studied. Ascorbic acid and potassium citrate were used as reducing agents.
KEYWORDS:silver nanoparticles; silver toluenesulfonate; silver methanesulfonate; ethylene glycol; methyl cellosolve
Download this article as:| Copy the following to cite this article: Glushko V, Blokhina L, Sadovskaya N, Kozhukhov V. Nonaqueous preparation of stable silver nano particles dispersions from organic sulfonic acids. Orient J Chem 2016;32(2) |
| Copy the following to cite this URL: Glushko V, Blokhina L, Sadovskaya N, Kozhukhov V. Nonaqueous preparation of stable silver nano particles dispersions from organic sulfonic acids. Orient J Chem 2016;32(2). Available from: http://www.orientjchem.org/?p=15457 |
Introduction
Silver nano particles – class of material with significant differences in physical, chemical characteristics, optical, electromagnetic and catalytic properties if compared with macroparticles 1.
The scope of silver nano particles use is very extensive. They can be used as additive to coating for solar energy absorption, as chemical reaction catalysts, in antimicrobial and disinfecting reagents. Nanosilver additives give antibacterial properties to clothing, paints and other materials 2-7.
Theoretical analysis
Since nano particles have intermediate position between true solutions and macro systems, there are two general approaches to nano particles preparation – dispersing and condensation. Dispersing methods are based on grinding of macroscopic particles to nanosize. Condensation methods are associated with the formation of particles in phase transitions 8. In the sense of large-scale application, chemical methods are considered as the most promising methods for nano particles synthesis, where various metals-containing compounds are used as starting substances: metal carbonyls, organometallic compounds, carboxylic acid salts, etc. 9-10.
A considerable number of known methods for silver nano particles production are associated with carrying out chemical reactions in aqueous medium 7, 11, 12. However, introduction of nano particles into hydrophobic materials requires synthesis in nonaqueous media, more specifically in organic solvents 13.
Synthesis of silver nano particles in organic solvents is associated with a range of necessary conditions, required to carry out the experiment: precursors for nano particles synthesis must have a sufficiently high solubility in the selected type of solvent, reducing agents must exhibit a high reducing capability in the current system and obtained nano particles must be also stabilized in the dispersion.
Usually, to increase the stability of nano-sized state, the additional component is added to the reaction medium – stabilizer. Stabilization can be also achieved with the help of adsorbed radicals on the surface of the nanoparticles (so-called “tails”) of reducing agent or precursor – source of silver ions 14.
Some authors note that thiosulfates, thiols and sulfonic acids are the most effective stabilizers due to the large affinity to metals 14. According to 15, thiosulfate-stabilized silver nanoparticles are obtained by sodium dodecyl sulfate reduction with sodium borohydride. In the process of synthesis, nano particles are initially stabilized by borohydride anions, and then the anions are partially substituted by thiosulfate anions with the following reduction to thiols.
In the current study, the process of silver nano particles synthesis in nonaqueous media without the use of additional stabilizers was studied. Organic silver sulfosalts were used as a precursor – we assume that in this case full additional stabilization due to sulfonate groups, adsorbed on the surface of nano particles should take place.
Materials and Methods
The following reagents were used for silver nanoparticles synthesis:
Silver nitrate – GOST 1277-75 “chemically clean”; silver carbonate (99.9 %); p-toluenesulfonic acid “chemically clean” (99.9%); potassium citrate (99.8%); ascorbic acid (99.5%); methanesulfonic acid “chemically clean” 99.8%; ethylene glycol; ethylene glycol methyl ether “chemically clean”; isopropyl alcohol 99.9%; sodium hydroxide 99.8%.
The presence of silver nanoparticles was monitored with spectrophotometer Specord 250 PLUS. The size of silver nanoparticles was determined by Zetasizer nano series NT Malvern.
Silver p-toluenesulfonate synthesis
2.5 ml 10-2 M potassium hydroxide (0.4 g) in water solution was added at normal conditions to 5 ml 10-2 M p-toluenesulfonic acid (1.9 g) in water solution and stirred. After, 5 ml 10-1 M AgNO3 (1.7 g) in water solution was added to the volume. The stirring was further carried out for 1 hour and the precipitate was filtered and dried in a vacuum desiccator over calcium chloride.
Yield – 1.0 g of silver p-toluenesulfonate.
Silver methanesulfonate synthesis
50 ml of isopropyl alcohol was added to 30 ml 2*10-2 M of silver carbonate (5.5 g) in methanesulfonic acid solution. The resulting mixture was cooled to 00С. The precipitate was filtered, washed with cold isopropyl alcohol and dried in a vacuum desiccator.
Yield – 6.5 g of silver methanesulfonate.
Method 1.1
Solution of 0.00176 g of ascorbic acid in 10 ml of ethylene glycol (C = 10-3 M) was added to solution of 0.00278 g of silver p-toluenesulfonate in 20 ml of ethylene glycol methyl ether (C=5×10-3 M). The stirring was held for 10 minutes.
Method 1.2
Solution of 0.00176 g of ascorbic acid in 10 ml of ethylene glycol (C = 10-3 M) was added to solution of 0.00278 g of silver p-toluenesulfonate in 20 ml of ethylene glycol (C=5×10-4 M). The stirring was held for 10 minutes.
Method 1.3
Solution of 0.00176 g of ascorbic acid in 10 ml of ethylene glycol methyl ether (C = 10-3 M) was added to solution of 0.00556 g of silver p-toluenesulfonate in 20 ml of ethylene glycol methyl ether (C=10-3 M). The stirring was held for 15 minutes.
Method 1.4
Solution of 0.00176 g of ascorbic acid in 10 ml of ethylene glycol methyl ether (C = 10-3 M) was added to solution of 0.00556 g of silver p-toluenesulfonate in 20 ml of ethylene glycol (C=10-3 M). The stirring was held for 15 minutes.
Method 1.5
Solution of 0.00816 g of potassium citrate in 25 ml of ethylene glycol (C = 10-3 M) was added to solution of 0.0014 g of silver p-toluenesulfonate in 5 ml of ethylene glycol methyl ether (C=10-3 M). The stirring was held for 30 minutes at 65 °C.
Method 1.6
Solution of 0.00816 g of potassium citrate in 25 ml of ethylene glycol (C = 10-3 M) was added to solution of 0.0014 g of silver p-toluenesulfonate in 5 ml of ethylene glycol (C=10-3 M). The stirring was held for 30 minutes at 65 °C.
Method 2.1
Solution of 0.0017 g of potassium citrate in 10 ml of ethylene glycol (C = 10-3 M) was added to solution of 0.00406 g of silver methanesulfonate in 20 ml of ethylene glycol methyl ether (C=10-3 M). The stirring was held for 10 minutes.
Method 2.2
Solution of 0.0017 g of potassium citrate in 10 ml of ethylene glycol (C = 10-3 M) was added to solution of 0.00406 g of silver methanesulfonate in 20 ml of ethylene glycol (C=10-3 M). The stirring was held for 10 minutes.
Method 2.3
Solution of 0.00176 g of ascorbic acid in 10 ml of ethylene glycol methyl ether (C = 10-3 M) was added to solution of 0.00406 g of silver methanesulfonate in 20 ml of ethylene glycol methyl ether (C=10-3 M). The stirring was held for 10 minutes.
Method 2.4
Solution of 0.00176 g of ascorbic acid in 10 ml of ethylene glycol methyl ether (C = 10-3 M) was added to solution of 0.00406 g of silver methanesulfonate in 20 ml of ethylene glycol (C=10-3 M). The stirring was held for 10 minutes.
Method 2.5
Solution of 0.0081 g of potassium citrate in 25 ml of ethylene glycol methyl ether was added to solution of 0.0010 g of silver methanesulfonate in 5 ml of ethylene glycol. The stirring was held for 15 minutes at 65 °C.
Method 2.6
Solution of 0.0081 g of potassium citrate in 25 ml of ethylene glycol was added to solution of 0.0010 g of silver methanesulfonate in 5 ml of ethylene glycol. The stirring was held for 15 minutes at 65 °C.
The presence of silver nano particles was monitored with spectrophotometer Specord 250 PLUS. The size of silver nano particles was determined by Zetasizer nano series NT Malvern. Pictures of nano particles dispersions were made using scanning electron microscope Hitachi SU1510.
Results and Discussions
Silver nanoparticles synthesis using silver p-toluenesulfonate
In case of synthesis using silver p-toluenesulfonate, silver nanoparticles are obtained under soft conditions, this was confirmed by absorption spectroscopy. Nevertheless, we didn’t manage to obtain stable dispersions for 10-3 M and for 5×10-4 M concentrations of the initial silver salt. The color of the solutions tended to be yellow (1.2, 1.5, 1.6), yellow-green (1.3) and brown (1.1) but some (1.4) were beige and muddy. The absorption peak of nanosilver was in the range of 410-450 nm. It should be noted that reduction with ascorbic acid resulted in formation of wide peak, which indicates polydispersity of the sample. For example – method 1.1 – the absorption spectra (Figure 1) had an intense peak with maximum at 430 nm and a tail up at 600 nm, which means that the solution contains not only spherical nanoparticles.
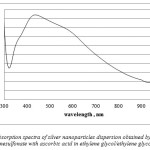 |
Figure 1: Absorption spectra of silver nanoparticles dispersion obtained by reduction of silver p- toluenesulfonate with ascorbic acid in ethylene glycol/ethylene glycol methyl ether |
In case of almost all methods (1.1, 1.2, 1.3, 1.4) the precipitation of silver occurred in one-two days – which indicates poor solution stability.
Better results were obtained using potassium citrate as the reducing agent. The dispersion obtained by method 1.5 was stable for one week and had a higher intensity peak in comparison with dispersions obtained by ascorbic acid reduction.
Dispersions with a good stability of more than 2 moths were obtained by silver p- toluenesulfonate reduction with potassium citrate in the medium of ethylene glycol (further, dispersion 1). The absorption spectra had a low intensity peak with maximum at 430 nm (Figure 2); nevertheless, the peak is very thin, which indicates the majority of spherical nanoparticles.
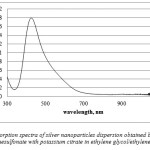 |
Figure 2: Absorption spectra of silver nanoparticles dispersion obtained by reduction of silver p- toluenesulfonate with potassium citrate in ethylene glycol/ethylene glycol system |
Silver nanoparticles synthesis using silver methanesulfonate
In case of silver methanesulfonate reduction with ascorbic acid in ethylene glycol medium we managed to obtain stable for at least 2 moths nanosilver dispersions (further, dispersion 2). The absorption spectra had a thin high intensity bond with maximum at 412 nm (Figure 3), which indicates the majority of spherical nanoparticles. The color of the solution was yellow with an opalescence effect. This dispersion is the most stable and considered as the best in case of silver methane sulfonate usage.
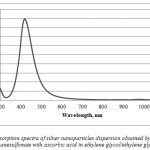 |
Figure 3: Absorption spectra of silver nanoparticles dispersion obtained by reduction of silver methanesulfonate with ascorbic acid in ethylene glycol/ethylene glycol system |
It should be noted that such results were only reached using ethylene glycol medium; the use of ethylene glycol methyl ether lead to precipitation of nanoparticles, in other words, the dispersion was unstable. With ethylene glycol methyl ether, the absorption spectra showed two peaks at 412 nm and 740 nm (Figure 4), which indicated polydispersity and presence of rod-shaped nanoparticle. The solution color was green and the dispersion coagulated in a day.
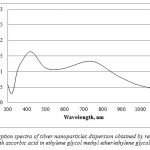 |
Figure 4: Absorption spectra of silver nanoparticles dispersion obtained by reduction of silver methanesulfonate with ascorbic acid in ethylene glycol methyl ether/ethylene glycol methyl ether system Click here to View figure |
In methods 2.4, 2.5, 2.6, solutions had high intensity peaks in the range of 410-430 nm but the reaction speed was very slow as color changed occurred only after 10 minutes of stirring. Method 2.4 produced stable for one week dispersions, whereas 2.2 and 2.6 dispersions were stable for three weeks without the following precipitation – solutions became transparent.
Much stable dispersions were obtained by silver methanesulfonate reduction with potassium citrate in ethylene glycol (further dispersion 3, Figure 5) and ethylene glycol/ethylene glycol methyl ether systems, resulting in high intensity peak at 450 nm. In ethylene glycol, the precipitation did not occur after two months– the solution color turned to transparent. With ethylene glycol/ethylene glycol methyl ether system the solution was stable for 3 weeks.
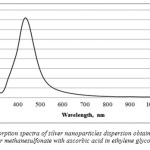 |
Figure 5: Absorption spectra of silver nanoparticles dispersion obtained by reduction of silver methane sulfonate with ascorbic acid in ethylene glycol system Click here to View figure |
The analysis of all absorption spectrums showed that dispersions obtained by silver methanesulfonate reduction had more narrow peaks than dispersions obtained by silver p-toluenesulfonate reduction. According to the comparison histogram of intensity of three best dispersions (dispersion 1-3), reduction of silver methanesulfonate reduction with ascorbic acid in ethylene glycol medium results in the maximum concentration of silver nanoparticles (Figure 6).
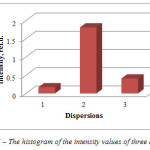 |
Figure 6: The histogram of the intensity values of three dispersions Click here to View figure |
Size distribution of nanoparticles of dispersion 1 is shown on Figure 7.
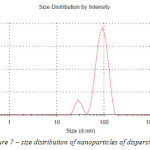 |
Figure 7: size distribution of nanoparticles of dispersion 1 Click here to View figure |
The average nano particle size for dispersion 1 is 95 nm. The amount of nano particles in the dispersion ranging from 25 to 70 nm is 8 %, from 70 to 95 nm – 48 %, from 95 to 130 nm – 44 %.
The results of nanoparticles size measurement by zeta-potential change correlates with the scanning electron microscopy (Figure 8).
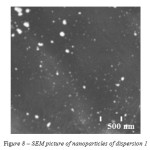 |
Figure 8: SEM picture of nanoparticles of dispersion 1 |
The microscopy confirms polydispersity – the sample contains a big amount of spherical nanoparticles of size less than 100 nm as well as bigger clusters of diameter more than 200 nm.
Zetasizer and SEM studies were carried out for dispersion 2 and 3 as well.
Figure 9 indicates that the distribution of silver nanoparticles is characterized by one clear peak – more than 70 % of nanoparticles are 70-120 nm; average size – 85 nm.
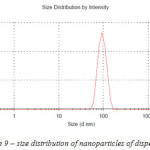 |
Figure 9: size distribution of nano particles of dispersion 2
|
The results of nano particles size measurement by zeta-potential change correlates with the scanning electron microscopy (Figure 10).
 |
Figure 10: SEM picture of nano particles of dispersion 2 Click here to View figure |
As dispersion 1, size distribution of nanoparticles of dispersion 3 has two peaks (Figure 11).
The average nanoparticle size for dispersion 3 is 93 nm. The amount of nanoparticles in the dispersion ranging from 8 to 10 nm is 2 %, from 70 to 97 nm – 54 %, from 97 to 120 nm – 44 %.
As for dispersion 1, the microscopy (Figure 12) of dispersion 3 confirms polydispersity – the sample contains a big amount of spherical nanoparticles of size less than 100 nm as well as bigger clusters of diameter more than 200 nm.
The study on the bacteriostatic activity showed that the growth of Escherichia coli and Staphylococcus aureus is inhibited by all specimens with an exposure for 30 min. The growth of pathogenic organisms after 24 and 48 hours is not revealed.
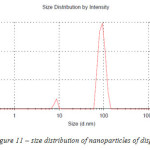 |
Figure 11:– size distribution of nanoparticles of dispersion 3 |
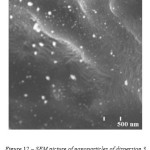 |
Figure 12: SEM picture of nanoparticles of dispersion 3 |
Conclusion
The most stable dispersions of silver nano particles are obtained by silver methanesulfonate reduction with ascorbic acid or silver p-toluenesulfonate reduction with potassium citrate in ethylene glycol medium. The resulting nano particles have a spherical form with a diameter of 85-97 nm. The dispersion obtained by methanesulfonate reduction with ascorbic acid has a narrower range of nano particles size distribution.
Accnowledgments
The applied researches are carried out with financial support of the state by the Russia Ministry of Education and Science under Grant Agreement No.14.576.21.0024 of June 27, 2014. (project № RFMEFI57614X0024).
References
- Pomogailo, A. D.; Rosenberg, A.C.; Ufland, I.E. Metal nano particles in polymers. M: Chemistry, 2000, 672
- Rao, C.N.R.; Muller, A.; Cheetham, A.K.; Colloid and Polymer Science. 2004, 283, 741
- Blagitko, E.M.; Burmistrov, V.A.; Kolesnikov, A. P. Silver in medicine. Novosibirsk: Science-Centre. 2004, 256
- Sosedova, L.M. Microelements in medicine. 2014, 15(4), 39−43
- Wiley, B.; Sun, Y.; Mayers, B.; Xia. Y. Chem. Eur. J. 2005, 11(2), 454-463
- Chen, G.; Wang, Y.; Yang, M.; Xu, J.; Goh, S. J.; Pan, M. J. Am. Chem. Soc. 2010, 132, 3644–3645.
- Egorova, E.M. Metal nanoparticles in solutions: biochemical synthesis, properties and application. Autoabstract, Moscow, 2011, 53.
- Summ, B.D.; Ivanova N.I. Colloidal-chemical aspects of nanochemistry – from Faraday to Prigogin. Moscow University Chemistry Bulletin. ser. 2. Chemistry. 2001, 42(5), 300-305
- Hyeon, T. Chem. Commun. 2003, 10, 927
- Sviridov, D.V. Photochemical, solvothermal and sonochemical synthesis of nano- and mesostructural materials. Bulletin BSU, ser. 2. 2011, 3, 12-15
- Hosseini, S. J.; Aghaie. H.; Ghaedi, M. Orient. J. Chem. 2014, 30(4), 1883
- Glushko, V.N.; Sadovskaya, N.Y.; Usova, O.A.; Blokhina, L.I.; Kozhukhov, V.I. Orient. J. Chem. 2015, 31(4), 2515-2520
- Olenin, A. U.; Lisichkin, G.V. М.: Uspekhi Khimii 2011, 80(7),635–736
- Shon, Y.S.; Cutler, E. Langmuir. 2004, 20(16), 6626–6630

This work is licensed under a Creative Commons Attribution 4.0 International License.









