Microwave Solvent-free Synthesis of Some Bioactive 3-(2,5-Dimethylfuran-3-yl)-pyrazoline Derivatives and Their Antimicrobial Activity
S. Kulathooran1, T. Vadivel1, M. Dhamodaran2* and B. Selvakumar3
1Research and Development Centre, Bharathiar University, Coimbatore-641046, India
2Department of Chemistry, Perunthalaivar Kamarajar Institute of Engineering and Technology, Karaikal-609603, India
3Department of Chemistry, PG and Research Centre, Sriparamakalyani College, Alwarkurichi 627412, India.
Corresponding author E-mail: dhamu_762003@yahoo.co.in
DOI : http://dx.doi.org/10.13005/ojc/320232
Article Received on :
Article Accepted on :
Article Published : 23 Apr 2016
A series of some new 1-thiocarbamoyl-3-(2,5-dimethylfuran-3-yl)-5-(fluoro/trifluoromethylphenyl)-2-pyrazolines 4a-f have been synthesized by treating with various fluoro/trifluoromethyl substituted chalcones, thiosemicarbazide and potassium carbonate using conventional heating and solvent-free microwave irradiation techniques. The easy work-up of the products, rapid reaction and mild conditions are noticeable features of this protocol. Synthesized compounds have been screened for their in vitro antimicrobial activity against six microbial strains. Among them, 1-Thiocarbamoyl-3-(2,5-dimethylfuran-3-yl)-5-(3-fluorophenyl)-2-pyrazoline 4b showed maximum zone of inhibition against all the tested microorganisms. Structural elucidation of the synthesized compounds were determined on the basis of various spectroscopic methods.
KEYWORDS:Microwave solvent-free reaction; Solid-phase synthesis; Chalcones; Pyrazolines; Antimicrobial activity
Download this article as:| Copy the following to cite this article: Kulathooran S, Vadivel T, Dhamodaran M, Selvakumar B. Microwave Solvent-free Synthesis of Some Bioactive 3-(2,5-Dimethylfuran-3-yl)-pyrazoline Derivatives and Their Antimicrobial Activity. Orient J Chem 2016;32(2) |
| Copy the following to cite this URL: Kulathooran S, Vadivel T, Dhamodaran M, Selvakumar B. Microwave Solvent-free Synthesis of Some Bioactive 3-(2,5-Dimethylfuran-3-yl)-pyrazoline Derivatives and Their Antimicrobial Activity. Orient J Chem 2016;32(2). Available from: http://www.orientjchem.org/?p=15441 |
Introduction
Increasing evidence suggests that pyrazoline derivatives possess a broad spectrum of biological activities antibacterial1-2, antifungal3-4 and pharmacological activities such as anti-inflammatory5-6, antitubercular7, anticancer8, analgesic9, antipyretic10 and anticonvulsant11. Based on the above study, we need to develop new drug against antimicrobial activities. Therefore, we were led to identify new approaches of pyrazoline derivatives as well as test the antimicrobial activity. Typically, synthesis of organic compounds using conventional method are found to take several hours to days to complete but with microwave assisted organic synthesis it takes few minutes12. The wide applicability of microwave activation in the chemical reactions is due to cleaner products, higher yield, shorter reaction time, operational simplicity, safe and minimization of side reactions13-14. In recent years the microwave reaction conditions on a inorganic base support is a promising alternative to conventional methods as these reactions represent a clean, efficient, economical and eco-friendly procedure15.
In continuation of our interest in the synthesis of various pyrazoline derivatives using conventional heating and microwave solvent-free techniques16 by telescopic method, it was our effort to develop an efficient method for synthesis of pyrazoline derivatives by using an inexpensive, safe, simple and common reagent. Alkali metal carbonates are weak bases and they are nontoxic in nature. Thus, the development of new methods that lead to convenient procedures with very short time period and better yields are of interest. Under the framework of green chemistry, we have developed an environmentally benign solvent-free approach for the synthesis of pyrazoline derivatives using potassium carbonate as an effective, economical and eco-friendly base catalyst. This expeditious and solvent free approach involves the exposure of neat reactants to microwave (MW) irradiation in conjunction with the use of supported reagents17.
Experimental
Melting points were determined using a Büchi apparatus. IR spectra were recorded for KBr disc on a Mattson 5000 FTIR spectrometer. All of the commercial chemicals and solvents were of reagent grade and were used without further purification. 1H and 13C NMR spectra were measured on a Bruker WP 400/75 MHz in DMSO-d6as solvent, using tetramethylsilane as internal standard, chemical shifts are expressed as ppm and J values are given in Hz. Mass spectrometric data were determined using an Agilent 6890 series instrument. The progress of the reaction was monitored by TLC using aluminum silica gel plates 60 F245. Products were purified by column chromatography using silica gel (60-120 mesh).
General procedure for the synthesis of 3-acetyl-2,5-dimethylfuran (2)
A stirred mixture of acetylacetone (2g, 0.02 mole) in toluene (5 ml), propargyl bromide (2.37g, 0.02 mole), 1,8-diazabicyclo[5.4.0]undec-7-ene (6.08g, 0.04 mole) and a catalytic amount of CuI (0.37g, 0.002 mole) was heated to 90 oC under nitrogen atmosphere for 5 hrs. Solvents were removed and the residue was subjected to the column chromatography using silica gel eluting with 10% mixture of ethyl acetate and n-heptane to obtain a title compound (2), which was confirmed by IR, 1H, 13C NMR and mass spectral studies18. 1H-NMR (400 MHz, DMSO-d6, δ, ppm): 2.21 (s, 3H, CH3), 2.25 (s, 3H, CH3), 2.42 (s, 3H, -COCH3), 6.09 (s, 1H, furyl-H). 13C NMR (75.46 MHz, DMSO-d6, δ, ppm) 189.42, 159.14, 152.70, 124.40, 106.24, 34.65, 14.64, 13.42. IR (KBr, ν cm-1): 1664 (C=O). MS (ESI) m/z: 139.2 (M+H)+.
General procedure for the synthesis of pyrazoline derivatives 4(a-f) using conventional heating method
A mixture of potassium carbonate (5g, 0.0362 mole) in water (2 mL), minimal amount of ethanol (1 mL), 3-acetyl-2,5-dimethylfuran (2g, 0.0144 mole) and 2-fluorobenzaldehyde (1.8g, 0.0144 mole) was stirred at room temperature (29 °C) for 10 minutes. Temperature of the reaction mass was increased to 45-50 °C and stirred vigorously for 1-2 h. Progress of the reaction was monitored by TLC and cooled to RT. To this mixture, thiosemicarbazide (1.6g, 0.0173 mole) and ethanol (10 ml) was added and stirred at refluxtemperature for 5-6 h. Progress of the reaction was monitored by TLC and cooled to RT. To the reaction mixture, ice-cold water was added and stirred well to remove the potassium carbonate present in the reaction mixture. The reddish yellow 1-Thiocarbamoyl-3-(2,5-dimethylfuran-3-yl)-5-(2-fluorophenyl)-2-pyrazoline was filtered at the pump, washed with ice-cold water and crystalized from ethanol. The above general method was adopted for the synthesis of compounds 4b-f of this series.
General procedure for the synthesis of pyrazoline derivatives 4(a-f) under the solvent-free microwave irradiation method
A mixture of 2-fluorobenzaldehyde (0.45g, 0.0036 mole), 3-acetyl-2,5-dimethylfuran (0.5g, 0.0036 mole) and solid K2CO3 (1.24g, 0.0090 mole) was ground with a mortar and pestle at room temperature. Further, it was subjected to microwave irradiation at 600 W in sealed vessel for 2-3 minutes, monitoring the progress of reaction by TLC. To this was, added thiosemicarbazide (0.40g, 0.0044 mole), stirred with glass rod at room temperature (29 °C) and subjected to microwave irradiation at 600 W in sealed vessel for 5-8 minutes, monitoring the progress of reaction by TLC. The reaction mixture was quenched with ice-cold water and filtered at the pump, followed by wash with ice-cold water and crystalized from ethanol to obtain reddish yellow 1-Thiocarbamoyl-3-(2,5-dimethylfuran-3-yl)-5-(2-fluorophenyl)-2-pyrazoline. The above general method was adopted for the synthesis of compounds 4b-f of this series.
Determination of antimicrobial activity
The antimicrobial activity of the synthesized compounds were evaluated by Agar well-diffusion method19-20. All the compounds were tested at different concentration level, DMSO was used as both solvent and control. The inhibition zone diameter in mm (IZD) was used as a criterion for the antimicrobial activity. The lowest concentration required to arrest the growth of microorganism was regarded as minimum inhibitory concentration (MIC, µg/mL) were determined for all the compounds and compared with the reference standard. The antimicrobial activities were assayed biologically using diffusion plate technique. The experiments were done by pouring a spore suspension 106 colon-forming units (CFU) per mL of the test strain to 75 mL of nutrient agar medium at 45 °C and mixed well, and poured into a 15 cm sterile metallic petri plate. The medium was allowed to solidify and 8 mm wells were dug with a sterile metallic borer, then DMSO solution of the test sample (1 mL) at 1 µg/mL was added to the respective wells. DMSO was used as negative control. The layer was allowed to set for 30 minutes and incubated at 37 °C for 48 h and results were noted.
Results and Discussion
3-acetyl-2,5-dimethylfuran was synthesized from commercially available acetylacetone. 3-Acetyl-2,5-dimethylfuran in a Claisen-Schmidt condensation with various fluoro/trifluoromethylbenzaldehydes and ethanol in the presence of K2CO3 using conventional heating and solvent-free microwave techniques to obtain desired chalcones. Further, it was treated with thiosemicarbazide without work-up and purification as a telescopic process to obtain 1-thiocarbamoyl-3-(2,5-dimethylfuran-3-yl)-5-(fluoro/trifluoromethylphenyl)-2-pyrazolines 4a-f using conventional heating and solvent-free microwave irradiation techniques21. The reaction time required for the synthesis of compounds 4a-f by the conventional method was 5-6 h, whereas the reaction time has been drastically brought down to 5-8 minutes with improved yields under solvent free microwave irradiation condition. Comparative studies were performed between conventional heating and solvent-free microwave methods and the results were presented in Table 1.
Table 1: Synthesis of pyrazoline derivatives 4a-f by conventional and microwave irradiation techniques
|
Compound |
Conventional |
MW |
Mpb (°C) |
||
|
Time |
Yielda (%) |
Time (min) |
Yielda (%) |
||
|
4a |
5 h |
59 |
5 |
73 |
176-178 |
|
4b |
5 h |
63 |
5 |
81 |
138-140 |
|
4c |
5 h |
54 |
5 |
76 |
178-180 |
|
4d |
5 h |
56 |
5 |
84 |
139-140 |
|
4e |
5 h |
64 |
5 |
69 |
174-176 |
|
4f |
5 h |
61 |
5 |
78 |
164-166 |
a Isolated yield b Uncorrected
Newly synthesized pyrazoline derivatives 4a-f were characterized by IR, 1H, 13C and mass spectral studies. The structures of pyrazolines were assigned based on detailed spectroscopic analysis. The IR spectrum of the compounds afforded pyrazoline C=N stretching (1569-1600 cm-1), thiocarbamoyl group N-H stretching (3458-3247 cm-1), C4-H deformation (1367-1483), C5-N1 stretching (1064-1149) and thiocarbamoyl group C=S stretching 1324-1358 cm-1 bands. In the 1H NMR spectra of the compounds HA, HB and HX protons of the pyrazoline ring were observed as doublet of doublet at δ 2.96-3.07 (JAB: 17.19-17.73 Hz), 3.83-3.96 (JAX: 4-10 Hz) and 5.83-6.10 ppm (JBX: 11.61-11.67 Hz), respectively. NH2 protons of the thiocarbamoyl group were seen at 7.54-8.02 ppm generally as broad bands, along with resonance in its 13C NMR at δ 175.36 indicating the presence of C=S function. In the mass spectra of the compounds molecular ion (M+) and M+2 were observed.
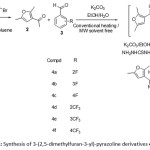 |
Scheme 1: Synthesis of 3-(2,5-dimethylfuran-3-yl)-pyrazoline derivatives 4a-f |
Antibacterial activity
The antibacterial activities of the synthesized compounds 4a-f were determined in vitro against three bacterial strains Klebsiella pneumonia ATCC-13883, Staphylococcus aureus ATCC-25923 and Escherichia coli ATCC-9637 at 50 µg/mL. The zone of inhibition was measured in mm and Ciprofloxacin used as standard antibacterial substance, under similar conditions for comparison. All the synthesized compounds have shown moderate to good activity against the tested microorganisms (Table 2). Among all, compound 4b shown maximum zone of inhibition against K. Pneumonia, S. Aureus and E. coli compared with their reference standard. Compound 4d and 4e also showed excellent potent against K. Pneumonia. Similarly, compound 4c, 4e and 4f also showed significant activity against E. coli. It concludes that, changing the fluoro and trifluoromethyl substitution in phenyl ring doesn’t provide any significant changes in antibacterial activity except fluorine substituted in m-position of phenyl ring.
Antifungal activity
The antifungal activity of the compounds 4a-f were tested against three pathogenic fungi, Candida albicans ATCC-28366, Rhizopus arrhizus ATCC-11145 and Aspegillus niger ATCC-26036 at 50 µg/mL concentration and the results were compared with Fluconazole as a reference standard. All the synthesized compounds have shown moderate to good activity against the tested microorganisms (Table 2). Among all, compound 4b shown maximum zone of inhibition against Candida albicans and Aspegillus niger. We observed that, fluorine substitution in m-position of phenyl ring observed significant activity than other compounds.
The lowest concentration required to arrest the growth of microorganism was regarded as minimum inhibitory concentration (MIC, µg/mL) were determined for all the compounds 4a-f and compared with the reference standard. The result of the MIC determinations reported in Table 2 showed that compound 4b exhibited broad spectrum action against K. Pneumonia, S. aureus, E. coli and C. Albicans and A. Niger compared with the reference standard. Compound 4c showed significant activity with MIC values of 1.9 and 7.2 µg/m Lagainst E. coli and R. Arrhizus, respectively. Compounds 4d and 4f displayed high activity against K. pneumonia, S. Aureus with MIC values of 8.7 and 7.4µg/mL, respectively.
Table 2: Antimicrobial activity of isolated products 4a-f
|
Compounds |
Inhibition zone in mma and MIC in µg/mL |
|||||||||||
|
K. Pneumonia |
S. Aureus |
E. coli |
R. Arrhizus |
C. Albicans |
A. Niger |
|||||||
|
A |
MIC |
A |
MIC |
A |
MIC |
A |
MIC |
A |
MIC |
A |
MIC |
|
|
4a |
18 |
23.2 |
11 |
56.7 |
8 |
>100 |
13 |
54.8 |
– |
– |
9 |
>100 |
|
4b |
26 |
4.7 |
28.2 |
2.9 |
22 |
6.7 |
11 |
56.2 |
21 |
2.2 |
28 |
1.3 |
|
4c |
21 |
43.2 |
20 |
44 |
25 |
1.9 |
19 |
7.2 |
18 |
14.3 |
12 |
59.2 |
|
4d |
29 |
1.4 |
18 |
>100 |
19 |
28.6 |
8 |
>100 |
11 |
>100 |
7 |
>100 |
|
4e |
27 |
2.9 |
12 |
96 |
25 |
1.8 |
18 |
12.1 |
9 |
>100 |
3 |
>100 |
|
4f |
14 |
>100 |
21 |
35.6 |
20 |
19.2 |
16 |
0 |
7 |
69 |
8 |
47 |
|
Ciprofloxacin |
30 |
0.86 |
30 |
0.06 |
26 |
1.2 |
– |
– |
– |
– |
– |
– |
|
Fluconazole |
– |
– |
– |
– |
– |
– |
24 |
0.72 |
21 |
2.9 |
26 |
0.3 |
Analytical Data
1-Thiocarbamoyl-3-(2,5-dimethylfuran-3-yl)-5-(2-fluorophenyl)-2-pyrazoline (4a)
1H NMR (300 MHz, DMSO-d6, δ, ppm): 2.20 (s, 3H, CH3), 2.41 (s, 3H, CH3), 2.97 (1H, dd, pyrazoline HA, JAB: 17.19 Hz, JAX: 3.61 Hz), 3.87 (1H, dd, pyrazoline HB, JAB: 17.6 Hz, JBX: 11.61 Hz), 5.96 (1H, dd, pyrazoline HX, JAx: 3.6 Hz, JBX: 11.61 Hz), 6.97 (s, 1H, furyl-H), 6.95-6.97 (m, 1H, Ar-H), 7.00-7.28 (m, 3H, Ar-H), 7.57 (b, 1H, NH), 7.95 (b, 1H, NH). 13C NMR (75.46 MHz, DMSO-d6, δ, ppm): 173.20, 159.50, 151.21, 150.23, 149.34, 129.04, 128.54, 124.53, 115.24, 113.21, 108.46, 106.42, 56.31, 47.56, 14.71, 13.41. IR (KBr, ν cm-1): 3387, 1582, 1478, 1401, 1365, 1096 cm-1. HR mass Calcd. for C16H16FN3OS: 317.28. Found: m/z 318.3 (M+H)+.
1-Thiocarbamoyl-3-(2,5-dimethylfuran-3-yl)-5-(3-fluorophenyl)-2-pyrazoline (4b)
1H NMR (300 MHz, DMSO-d6, δ, ppm): 2.19 (s, 3H, CH3), 2.41 (s, 3H, CH3), 2.97 (1H, dd, pyrazoline HA, JAB: 17.73 Hz, JAX: 3.61 Hz), 3.83 (1H, dd, pyrazoline HB, JAB: 17.6 Hz, JBX: 11.61 Hz), 5.86 (1H, dd, pyrazoline HX, JAx: 3.6 Hz, JBX: 11.61 Hz), 6.37 (s, 1H, furyl-H), 6.86-6.94 (m, 2H, Ar-H), 7.01-7.06 (m, 1H, Ar-H), 7.31-7.38 (m, 1H, Ar-H), 7.58 (b, 1H, NH), 7.97 (b, 1H, NH). 13C NMR (75.46 MHz, DMSO-d6, δ, ppm): 174.90, 162.50, 151.80, 150.23, 149.70, 145.10, 130.24, 122.04, 114.24, 113.51, 113.12, 107.26, 66.31, 47.56, 14.71, 13.41. IR (KBr, ν cm-1): 3482, 1595, 1483, 1449, 1363, 1008 cm-1. HR mass Calcd. for C16H16FN3OS: 317.28. Found: m/z 319.3 (M+H)2+.
1-Thiocarbamoyl-3-(2,5-dimethylfuran-3-yl)-5-(4-fluorophenyl)-2-pyrazoline (4c)
1H NMR (300 MHz, DMSO-d6, δ, ppm): 2.20 (s, 3H, CH3), 2.41 (s, 3H, CH3), 2.97 (1H, dd, pyrazoline HA, JAB: 17.6 Hz, JAX: 3.67 Hz), 3.83 (1H, dd, pyrazoline HB, JAB: 17.6 Hz, JBX: 11.64 Hz), 5.83 (1H, dd, pyrazoline HX, JAx: 3.6 Hz, JBX: 11.64 Hz), 6.37 (s, 1H, furyl-H), 7.11-7.13 (m, 4H, Ar-H), 7.54 (b, 1H, NH), 7.91 (b, 1H, NH). 13C NMR (75.46 MHz, DMSO-d6, δ, ppm): 175.90, 160.90, 151.81, 150.73, 149.74, 139.14, 128.60, 115.53, 115.24, 113.12, 107.16, 106.42, 66.13, 41.56, 14.71, 13.41. IR (KBr, ν cm-1): 3479, 1600, 1481, 1420, 1358, 1084 cm-1. HR mass Calcd. for C16H16FN3OS: 317.28. Found: m/z 318.1 (M+H)+.
1-Thiocarbamoyl-3-(2,5-dimethylfuran-3-yl)-5-(2-(trifluoromethyl)phenyl)-2-pyrazoline (4d)
1H NMR (300 MHz, DMSO-d6, δ, ppm): 2.20 (s, 3H, CH3), 2.42 (s, 3H, CH3), 2.96 (1H, dd, pyrazoline HA, JAB: 17.6 Hz, JAX: 3.61 Hz), 3.96 (1H, dd, pyrazoline HB, JAB: 17.5 Hz, JBX: 11.67 Hz), 6.10 (1H, dd, pyrazoline HX, JAx: 3.6 Hz, JBX: 11.67 Hz), 6.38 (s, 1H, furyl-H), 7.44-7.47 (m, 1H, Ar-H), 7.59-7.63 (m, 2H, Ar-H), 7.65 (b, 1H, NH), 7.72-7.74 (m, 1H, Ar-H), 8.02 (b, 1H, NH). 13C NMR (75.46 MHz, DMSO-d6, δ, ppm): 175.20, 151.81, 150.73, 149.74, 138.01, 131.94, 127.54, 127.30, 125.81, 125.01, 119.14, 107.26, 106.42, 59.61, 41.41, 14.71, 13.41. IR (KBr, ν cm-1): 3450, 1579, 1483, 1422, 1306, 1057cm-1. HR mass Calcd. for C17H16F3N3OS: 367.1. Found: m/z 368.1 (M+H)+.
1-Thiocarbamoyl-3-(2,5-dimethylfuran-3-yl)-5-(3-(trifluoromethyl)phenyl)-2-pyrazoline (4e)
1H NMR (300 MHz, DMSO-d6, δ, ppm): 2.22 (s, 3H, CH3), 2.44 (s, 3H, CH3), 3.07 (1H, dd, pyrazoline HA, JAB: 17.7 Hz, JAX: 3.67 Hz), 3.91 (1H, dd, pyrazoline HB, JAB: 17.9 Hz, JBX: 11.61 Hz), 5.98 (1H, dd, pyrazoline HX, JAx: 3.67 Hz, JBX: 11.61 Hz), 6.40 (s, 1H, furyl-H), 7.39-7.46 (m, 2H, Ar-H), 7.55-7.60 (m, 3H, Ar-H), 7.62 (b, 1H, NH), 7.99 (b, 1H, NH). 13C NMR (75.46 MHz, DMSO-d6, δ, ppm): 175.90, 151.80, 150.73, 149.74, 143.81, 130.84, 130.13, 128.94, 124.90, 125.81, 124.51, 113.14, 107.26, 66.41, 41.41, 14.71, 13.41. IR (KBr, ν cm-1): 3479, 1569, 1482, 1348, 1320, 1073 cm-1. HR mass Calcd. for C17H16F3N3OS: 367.1. Found: m/z 368.1 (M+H)+.
1-Thiocarbamoyl-3-(2,5-dimethylfuran-3-yl)-5-(4-(trifluoromethyl)phenyl)-2-pyrazoline (4f)
1H NMR (300 MHz, DMSO-d6, δ, ppm): 2.22 (s, 3H, CH3), 2.43 (s, 3H, CH3), 3.07 (1H, dd, pyrazoline HA, JAB: 17.6 Hz, JAX: 3.7 Hz), 3.91 (1H, dd, pyrazoline HB, JAB: 17.7 Hz, JBX: 11.64 Hz), 5.95 (1H, dd, pyrazoline HX, JAx: 3.6 Hz, JBX: 11.64 Hz), 6.39 (s, 1H, furyl-H), 7.32-7.34 (m, 2H, Ar-H), 7.62 (b, 1H, NH), 7.68-7.70 (m, 2H, Ar-H), 7.99 (b, 1H, NH). 13C NMR (75.46 MHz, DMSO-d6, δ, ppm): 175.90, 151.81, 150.71, 149.71, 146.80, 129.10, 127.34, 127.14, 125.03, 125.49, 124.21, 113.10, 107.26, 66.61, 41.10, 14.71, 13.41. IR (KBr, ν cm-1): 3444, 1594, 1478, 1367, 1324, 1064 cm-1. HR mass Calcd. For C17H16F3N3OS: 367.1. Found: m/z 368.1 (M+H)+.
Conclusion
In conclusion, we have developed a simple, rapid and efficient green synthesis of 1-thiocarbamoyl-3-(2,5-dimethylfuran-3-yl)-5-(fluoro/trifluoromethylphenyl)-2-pyrazolines from commercially available acetylacetone in good yield under mild reaction condition. Microwave irradiation solvent-free method shortened the reaction time and improves yield of products (5-8 min. and 69-84%) than conventional method (5-6 hr and 54-64%). The synthesized compounds were evaluated for their in vitro antimicrobial activity against various pathogenic bacterial and fungal strains. Compound 4b showed valuable inhibitory activity against most of the microbial strains compared with standards.
Acknowledgments
The authors are gratefully acknowledged to Hi-Tech research foundation, Tharangambadi, Tamilnadu, India for providing the financial and laboratory facilities. Authors also thank Sophisticated Test & Instrumentation Centre, Cochin University of Science and Technology, Cochin, Kerala, India for spectral studies.
References
- Sangapure, S. S., Bodke, Y., Raga, B., Ind. J. Heterocycl. Chem., 2001, 11, 31–37.
- Rishiram, P., Janmajoy, B., Hemanta, K., Orient. J. Chem., 2015, 31(4), 2099-2106.
CrossRef - Gupta, U., Sareen, V., Khatri, V., Chug, S., Ind. J. Heterocycl. Chem., 2005, 14, 265–266.
- Ashish, K.T., Anil, M., Verma, H. N., Mishra, A., Ind. J. Chem.,2006, 45B, 489–492.
- Sivasanker, R.L., Rajkumar, T., Lakshmi, M.G., Sivarami, R.Y., Orient. J. Chem., 2015, 31(Spl Edn.), 189-199.
- Makhsumov, A. D., Dzhurae, K. G., Nikbae, A. T., Pharm. Chem. J., 1986, 20, 289–291.
- Chetan, B.P., Mulwar, V.V., Ind. J. Chem.,2000, 44B, 232–237.
- Nimavat,K.S., Popat, K.H., Ind. J. Heterocycl. Chem.,2007, 16, 333–336.
- Udupi, R.H., Bhat, A.R., Krishna, K., Ind. J. Heterocycl. Chem., 1998, 8, 143–146.
- Fabiane, R.S., Vanessa, T.S., Viviane, R., Lysandro, P.B., Marli, R.O., Helio, G.B., Nilo, Z., Marcos, A. P. M., Carlos, F. M., Eur. J. Pharmacol., 2002, 451, 141–147.
CrossRef - Ashok, K., Archana, Sharma, S.,Ind. J. Hetero. Chem., 2001, 9, 197.
- Varma, R. S., Microwaves in Organic Synthesis (A. Loupy, Ed.), Wiley-VCH, Weinheim 2002. 6, 181-218,
- Hisashi, A., Koichi, F., Shoichi, K., Synlett, 2004, 6, 1049-1053.
- Ameta, K. L., Varma, B. L., Indian Chem. Soc.,2002, 79, 840-841.
- Mondal, R., M., Gupta, A. D., Mallik, A. K.,Tetrahedron Lett., 2011, 52, 5020-5024.
CrossRef - Varma, R. S., Pure Appl. Chem.,2001, 73(1), 193-198.
CrossRef - Varma R. S., Tetrahedron.,2002, 58(7), 1235-1255.
CrossRef - Kulathooran, S., Selvakumar, B.,Dhamodaran, M., Der. Pharma. Chemica. 2014, 6(3), 240-249.
- Schelz, Z.,Molnar, J., Hohmann. J., Fitoterapia 2006, 77, 279-285.
CrossRef - Essam, M. S.; Nagwa, M. M. H. Am. J. Org. Chem. 2012, 2, 26-31.
CrossRef - Rishikesh, V. A., Cendilkumar, A., Divakar, G., Ganesh, S. A., Rajesh, J. O., Saudi Pharm J., 2011, 19, 233-243.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









