In vitro screening of subtropical plants cultivated in Jeju Island for cosmetic ingredients
Min-Jin Kim1‡, Ju Mi Hyun1‡, Sang Suk Kim2, Ki Cheol Seong3, Chan Kyu Lim3, Jong-Seok Kang1, Suk Hyun Yun2, Kyung Jin Park2, Hyun Joo An2, Kyo Sun Park3, Young Hun Choi2, Nam Ho Lee1, and Chang-Gu Hyun1*
1Cosmetic Science Center, Department of Chemistry and Cosmetics, Jeju National University, Jeju 63243, Korea 2Citrus Research Institute, National Institute of Horticulture and Herbal Science, RDA, Seogwipo 63607, Korea 3Agricultural Research Institute for Climate Change, National Institute of Horticulture and Herbal Science, RDA, Jeju 63240, Korea. Corresponding author email: cghyun@jejunu.ac.kr
DOI : http://dx.doi.org/10.13005/ojc/320206
Article Received on :
Article Accepted on :
Article Published : 09 May 2016
To identify novel cosmetic ingredients from subtropical plants, we screened 21 parts of 12 plant species collected from the Agricultural Research Institute for Climate located in Jeju Island, the southernmost island of the Korean Peninsula. Subtropical plants were investigated for their total polyphenolic content by using the Folin-Ciocalteu reagent with gallic acid as the standard as well as 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS) scavenging activities. In both the DPPH and the ABTS assays, three plants, Syzygium samarangense, Acca sellowiana (leaves and branches), and Olea europaea (branches) showed significantly greater scavenging activity [half-maximal inhibitory concentration (IC50) < 50 μg/mL] than the other plants did. The leaves and branches of S. samarangense also had the highest total phenolic content (153.7 and 160.6 mg gallic acid equivalent [GAE]/g DW, respectively). However, the subtropical plants in this study showed lower elastase and tyrosinase inhibition activities than the positive controls oleanolic acid and arbutin (95.0 and 86.5 μg/mL, respectively) did. Furthermore, we sought to investigate the anti-inflammatory effects of these subtropical plants for potential use in topical applications to treat skin inflammation. Therefore, they were screened for inhibitory effects on the proinflammatory mediator, nitric oxide (NO) in lipopolysaccharide (LPS)-stimulated macrophage RAW 264.7 cells. Our results revealed that S. samarangense leaves potently inhibited the LPS-stimulated NO production concentration-dependently with an IC50 of 154.3 μg/mL. These results suggest that these subtropical plants possess several biological activities that may be potent inhibitors of the skin aging and inflammatory processes. Further investigations will focus on cell-based in vitro assays and chemically identifying the major active components mediating the anti-aging and anti-inflammation.
KEYWORDS:ABTS; DPPH; Cosmetic; Elastase; Jeju Island; Subtropical plants; Tyrosinase
Download this article as:| Copy the following to cite this article: Kim M. J, Hyun J. M, Kim S. S, Seong K. C, Lim C. K, Kang J. S, Yun S. H, Park K. J, An H. J, Park K. S, Choi Y. H, Lee N. H, Hyun C. H. In vitro screening of subtropical plants cultivated in Jeju Island for cosmetic ingredients. Orient J Chem 2016;32(2) |
| Copy the following to cite this URL: Kim M. J, Hyun J. M, Kim S. S, Seong K. C, Lim C. K, Kang J. S, Yun S. H, Park K. J, An H. J, Park K. S, Choi Y. H, Lee N. H, Hyun C. H. In vitro screening of subtropical plants cultivated in Jeju Island for cosmetic ingredients. Orient J Chem 2016;32(2). Available from: http://www.orientjchem.org/?p=15962 |
Introduction
Skin aging is a biological process that induces changes in the structural integrity and physiological function of the skin. Exposure to ultraviolet (UV) radiation is one of the most significant external stress-inducing factors and a major cause of premature skin aging. Wrinkle formation is a striking feature of intrinsic and photo-induced skin aging, which are both associated with oxidative stress and inflammatory responses. Skin aging is characterized by the progressive loss of structural integrity and physiological function of the skin and is caused by intrinsic and extrinsic factors that lead to senescence and degradation of the biological function of the skin. The natural aging process induces, the skin wrinkling, which is accompanied by the production of excessive amounts of reactive oxygen species (ROS)1-4. The over-accumulation of free radicals causes numerous harmful effects to the skin through the activation of wrinkles-related enzymes such as elastase and collagenase, which further contribute to skin aging5-6. Whereas tyrosinase is a copper-containing enzyme, which is responsible for skin pigmentation while the over-accumulation of melanin in specific parts of the skin results in undesirable skin hyperpigmentation such as sunburn, liver spots, and freckles7-8.
Natural-occurring secondary metabolites provide a largely unexplored source for the potential development of new drugs. Indeed, plant extracts have received considerable attention as potential candidate ingredients in several cosmetic applications. Some promising skin applications for natural-based ingredients include anti-wrinkling agents through elastase inhibition and antioxidant mechanisms, skin whitening agents through mushroom tyrosinase inhibition, and anti-irritation agents through nitric oxide inhibition9. Several studies have reported the use of plant extracts either singly or in combinations as cosmetic ingredients such as extracts of Portulaca oleracea, Carthamus tinctorius (safflower), Euphorbia supina, Malpighia Glabra (anti-aging), Broussonetia kazinoki, Glycyrrhiza uralensis, Morus alba, Morus bombycis (whitening activity), Hypochoeris radicata, Sasa quelpaertensis, and Acanthopanax koreanum (anti-irritant)10-12.
Over the past few years, we have systematically evaluated and characterized selected Jeju plants for their novel bioactivity or potential cosmetic application13. As part of the effort to discover new functional ingredients for skin whitening, anti-wrinkling, and anti-irritant preparations, we investigated the in vitro anti-aging, anti-tyrosinase, and anti-inflammatory activity of the extracts of 21 subtropical plants cultivated in Jeju Island.
Materials and Methods
Subtropical plant materials and extract preparation
The subtropical plants were collected from the Agricultural Research Institute for Climate located on Jeju Island in 2015. Voucher specimens were deposited at the Herbarium of the Cosmetic Science Center, Department of Chemistry and Cosmetics while the identity of the vouchers and fresh plants was verified by Dr. Ki Cheol Seong. The dried plant parts (i.e., the leaves branches, fruit, and aerial parts) were coarsely powdered by using an electric blender, extracted with 70% (v/v) ethanol, and were then stored at room temperature for 5 days. Then, the extracted solutions were filtered, the solvents were evaporated, and all the extracts obtained were dried and stored in an airtight container at 4°C. The dry extract yields are shown in Table 1.
Table 1: Extraction yields of Jeju subtropical plants
|
Numbers |
Scientific Names |
Parts |
Solvents |
Extracts |
Yield (%) |
|
1 |
Sechium edule |
leaves |
70% EtOH |
0.1072 |
10.7 |
|
2 |
Momordica charantia L. |
leaves |
70% EtOH |
0.1358 |
13.6 |
|
3 |
flesh |
70% EtOH |
0.1519 |
15.2 |
|
|
4 |
peel |
70% EtOH |
0.2966 |
29.7 |
|
|
5 |
Carica papaya L. |
leaves |
70% EtOH |
0.1157 |
11.6 |
|
6 |
fruit |
70% EtOH |
0.4054 |
40.5 |
|
|
7 |
Saccharum officinarum |
leaves |
70% EtOH |
0.108 |
10.8 |
|
8 |
Gynura bicolor |
aerial part |
70% EtOH |
0.1623 |
16.2 |
|
9 |
Syzygium samarangense |
leaves |
70% EtOH |
0.1453 |
14.5 |
|
10 |
branches |
70% EtOH |
0.0807 |
8.1 |
|
|
11 |
Passiflora edulis |
leaves |
70% EtOH |
0.2309 |
23.1 |
|
12 |
branches |
70% EtOH |
0.1321 |
13.2 |
|
|
13 |
Litchi chinensis |
leaf |
70% EtOH |
0.1433 |
14.3 |
|
14 |
Malpighia emarginata |
leaf |
70% EtOH |
0.1563 |
15.6 |
|
15 |
branches |
70% EtOH |
0.1136 |
11.4 |
|
|
16 |
Averrhoa carambola |
leaves |
70% EtOH |
0.2718 |
27.2 |
|
17 |
branches |
70% EtOH |
0.1172 |
11.7 |
|
|
18 |
Acca sellowiana |
leaves |
70% EtOH |
0.1459 |
14.6 |
|
19 |
branches |
70% EtOH |
0.1553 |
15.5 |
|
|
20 |
Olea europaea |
leaves |
70% EtOH |
0.2566 |
25.7 |
|
21 |
branches |
70% EtOH |
0.1946 |
19.5 |
Total polyphenol content
The total polyphenolic content was determined by using the Folin–Ciocalteu assay. Briefly, the extracts were mixed with 100 μL Folin–Ciocalteu reagent and incubated at room temperature for 5 min. Then, 0.2 mL of 7% sodium carbonate was added, and the absorbance of the reaction mixtures was measured at 720 nm by using a spectrophotometer. Gallic acid was used as a standard, and the total polyphenol content of each sample was expressed in milligram of gallic acid equivalent per gram (mg GAE/g) of extract.
2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Assay
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity of the subtropical plant extracts was measured as previously described with minor modifications13-15. Briefly, 180 μL of DPPH solution in 0.2 mM in ethanol was mixed with 20 μL of the sample in ethanol at different concentrations (10–500 μg/mL) and incubated in the dark at 25°C for 10 min. Then, the absorbance of the sample (Abssample) was measured at 517 nm as well as that of. The absorbance of a negative control (Abscontrol) consisting of only ethanol alone. The DPPH radical scavenging activity of the sample was calculated by using the following equation
DPPH scavenging activity = [(Abscontrol – Abssample)/Abscontrol] × 100
2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS) Radical Scavenging Assay
The 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS) radical scavenging activity of the subtropical plant extracts was measured as previously described with minor modifications13-15. Briefly, ABTS and potassium persulfate solutions (7 and 2.45 mM, respectively) were mixed and then incubated in the dark at 25°C for 16 h. The ABTS solution was diluted with ethanol to obtain the working solution with an absorbance at 700 nm (Abs700) of 0.78 ± 0.02. Then, 20-μL aliquots of the subtropical plant extract samples were dissolved in ethanol at different concentrations (10–500 μg/mL), mixed with 180 μL of the working solution, incubated in the dark at 25°C for 10 min, and then the Abssample and Abscontrol (ethanol only) were measured. The ABTS radical scavenging activity was calculated by using the following equation:
ABTS radical scavenging activity (%) = [(Abscontrol – Abssample)/Abscontrol] × 100
Tyrosinase inhibition assay
The mushroom tyrosinase inhibition activity was determined according to a method previously described in the literature with some modifications13-15. Briefly, mushroom tyrosinase (2500 units/mL) and L-tyrosine (0.07 mL) were added to a solution of phosphate buffer (0.1 M, pH 6.8, 0.09 mL) containing the test sample. The test mixture (0.2 mL) was incubated for 10 min at 37°C, and the absorption due to the formation of dopa-chrome was measured at 475 nm. A similar mixture without the plant extract and a solution of arbutin (hydroquinone-O-β-glucopyranoside) were used as the vehicle and positive controls, respectively. Each treatment was replicated three times, and the percent inhibition of the tyrosinase activity was calculated as follows:
inhibition (%) = [1 – (Abssample – Absblank)/Abscontrol] × 100
Where, Absblank is the absorbance of the blank while the half maximal inhibitory concentration (IC50) indicated the concentration of the subtropical plant extract sample that inhibited the enzyme activity by 50%, as determined by linear curve fitting.
Elastase inhibition assay
The porcine pancreatic elastase (PPE) inhibition activity was determined using N-Succ-(Ala)3-p-nitroanilide (SANA) as the substrate according to a previously reported method with some modifications13-15. Briefly, PPE (0.1 mg/mL, 0.01 mL) and SANA (6.5 mM, 0.005 mL) were added to Tris-hydrochloride (HCl) buffer (0.2 M, 0.165 mL) containing the test sample. The test mixture (0.2 mL) was incubated for 15 min at 25°C, and the absorption due to the formation of ρ-nitroaniline was measured at 405 nm. Then, similar mixtures without the subtropical plant extracts and a solution of oleanolic acid were used as the vehicle and positive controls, respectively. Each treatment was replicated three times, and the percentage (%) inhibition of elastase activity was calculated as follows:
inhibition (%) = [1 – (Abssample – Absblank)/Abscontrol] × 100
Cell culture
The RAW 264.7 murine macrophages were purchased from the Korean Cell Line Bank (Seoul, Korea) and were maintained at sub-confluence in a 5% CO2 humidified atmosphere at 37°C. The medium used for the routine subculture was Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS), penicillin (100 units/mL), and streptomycin (100 μg/mL).
Lactate dehydrogenase (LDH) assay for measuring cytotoxicity
The RAW 264.7 cells (1.8 × 105cells/mL) were plated in 24-well plates, preincubated for 18 h, treated with the indicated concentrations of the subtropical plant extracts for 2 h, and then challenged with lipopolysaccharide (LPS, 1μg/mL) for an additional 18 h. The extract cytotoxicity by using an LDH cytotoxicity detection kit (Promega, Madison, WI, USA) to determine the LDH release from the cells in the production of nicotinamide adenine dinucleotide phosphate (NADH) during the conversion of lactate to pyruvate. The optical density of the solution was measured at a wavelength of 490 nm.
Nitric oxide (NO) determination
The nitrite concentration of the cell culture medium was measured as an indicator of nitric oxide (NO) production according to the Griess reaction method. In brief, RAW 264.7 cells (1.8 × 105cells/mL) were plated in 24-well plates, incubated for 24 h, pretreated with the indicated concentrations of the subtropical plant extracts for 2 h, and then challenged with LPS (1 μg/mL) for an additional 18 h. Then, equal volumes of the culture medium and Griess reagent (1% sulfanilamide and 0.1% N-(1-naphthyl)-ethylenediamine dihydrochloride in 5 % phosphoric acid) were mixed for 10 min, and absorbance was measured at 540 nm.
Measurement of pro-inflammatory mediators
The cells (1.8 × 105cells/mL) were cultured in 24-well plates in the presence of various concentrations of the extract samples and LPS (1 μg/mL), and then incubated for 24 h. Then, the cell culture medium was centrifuged at 13,000 rpm for 10 min, and the supernatants were collected for the analysis of interleukin (IL)-6, IL-1β, tumor necrosis factor (TNF)-α, and prostaglandin E2 (PGE2) levels. This was performed using enzyme-linked immunosorbent assay (ELISA) kits (mouse IL-6 and TNF-α ELISA, Invitrogen Inc., mouse PGE2 and IL-1β ELISA, Abcam Inc.) following the recommendations of the manufacturers.
Data analysis
All the data were expressed as the means ± standard deviation (SD) of at least replicates. The Student’s t-tests and a one-way analysis of variance (ANOVA) were used for the statistical analyses, and differences with P-values < 0.05 were considered significant.
Results and Discussion
Extract yields and phenolic compounds analysis
The extract yields indicate that the extraction rates were dependent on the solubility of the various components of the 21 parts of the 12 subtropical plants in the 70% ethanol used as the extraction solvent. The highest and lowest extract yields were obtained with the Carica papaya fruits and Syzygium. samarangense at 40.5 and 8.1%, respectively. Numerous studies have reported that polyphenolic compounds are attractive ingredients for general and functional cosmetics because of their beneficial biological properties. Furthermore, the elucidation of the activities of polyphenol-containing plants has shown them to be effective in the prevention of wrinkles, melanin overproduction, and skin inflammation. Therefore, the total polyphenolic content of the subtropical plants extracts was assessed, and the results are presented in Table 2. The highest polyphenolic content was found in S. samarangense followed by Malpighia emarginata (both branches, 160.5 and 155.7 mg GAE/g, respectively).
Antioxidant activity
The principle of the antioxidant activity of a substance depends on the availability of electrons to neutralize any free radicals. In this study, the antioxidant activity of the subtropical plant extracts was evaluated by using the DPPH and ABTS+ radical scavenging assays. DPPH, a stable radical, is widely accepted as a suitable method for screening the free radical-scavenging ability of natural extracts and their compounds. The results of the radical scavenging assays for all the extracts are presented in Table 2 as the IC50 (mg/mL), which indicates the concentration of the test sample that efficiently scavenged half of the DPPH free radicals. The most active plants examined were S. samarangense (leaves), S. samarangense (branches), Acca sellowiana (leaves and branches), and O. europaea (branches) with IC50 values of 36.0, 48.1, 46.1, 7.2, and 44.2 μg/mL, respectively. The results imply that these active extracts may contain constituents with strong proton-donating abilities. ABTS, a protonated and relatively stable free radical, has characteristic absorbance maxima at 734 nm, which decreases with the scavenging of the proton radicals. Therefore, the antioxidant activity of the test plant extracts was also determined using the ABTS radical decolorization assay, which monitors the intensity of the green color of the reaction mixture that exhibits a proportional change to that of the antioxidant concentration. The results of this assay showed IC50 values in the range of 452.6–10.3 mg/mL. The highest ABTS radical scavenging activity was exhibited by seven plant extracts, S. samarangense leaves, S. samarangense branches, L. chinensis leaves, A. sellowiana leaves, A. sellowiana branches, O. europaea leaves, and O. europaea branches with IC50 values of 10.6, 10.3, 11.3, 10.3, 43.0, 44.0, and 48.4 μg/mL, respectively. Their activities were more than or comparable to those of butylated hydroxytoluene (BHT) and vitamin C (IC50 values, 23.0 and 8.2 μg/mL, respectively). These extracts showed that the ABTS radical scavenging activity exhibited a strong correlation with the total polyphenolic content.
Tyrosinase and elastase inhibition capacity
Tyrosinase expression and activation play an essential role in melanin production. Although melanin protects the skin against UV injury under normal physiological conditions, abnormal pigmentation such as freckles and age spots often causes serious skin problems and esthetic issues. Therefore, the modulation of melanogenesis through the regulation of tyrosinase and its related proteins is an important strategy for treating abnormal skin pigmentation13. A few known natural tyrosinase inhibitors of melanin synthesis including adenosine, arbutin, niacinamide, and kojic acid are currently used as cosmetic and medicinal ingredients for preparations used for the treatment of hyperpigmentation. Most skin-whitening ingredients that are currently available often have carcinogenic potentials as well as weak efficacies and, therefore, there is a clear need for the development of safer and more efficient ingredients. Table 2 summarizes the results of the assessment of mushroom tyrosinase inhibition by the 21 test plant extracts with their inhibition expressed as IC50 values, which reveal that they showed lower activities than the positive control arbutin (86.5 μg/mL) did. Furthermore, this study revealed that seven of the 21 extracts (S. samarangense leaves, S. samarangense branch, L. chinensis leaves, M. emarginata leaves, M. emarginata branches, A. sellowiana leaves, and A. sellowiana branches) had lower anti-tyrosinase activity compared to the positive controls arbutin (86.5 μg/mL) of IC50 values are 284.8, 1184.1, 1335.2, 1483.4, 1008.0, 1475.7, 550.7 respectively. We also investigated the 21 subtropical plant extracts for potential elastase inhibition and 13 extracts did not inhibit porcine pancreatic elastase (PPE) activity. Furthermore, we investigated the dose-response relationships of the eight plant extracts (S. samarangense leaves, S. samarangense branches, A. carambola leaves, A. carambola branches, A. sellowiana leaves, A. sellowiana branches, O. europaea leaves, and O. europaea branches) that showed biological activities, and the calculated IC50 values are presented in Table 2.
Table 2: Total polyphenol contents of Jeju subtropical plant extracts
|
Numbers |
Scientific Names |
Parts |
Total Polyphenol (mg/g GAE) |
|
|
1 |
Sechium edule |
leaves |
26.5 |
|
|
2 |
Momordica charantia L. |
leaves |
6.0 |
|
|
3 |
flesh |
7.1 |
||
|
4 |
peel |
6.9 |
||
|
5 |
Carica papaya L. |
leaves |
20.4 |
|
|
6 |
fruit |
3.5 |
||
|
7 |
Saccharum officinarum |
leaves |
27.5 |
|
|
8 |
Gynura bicolor |
aerial parts |
29.0 |
|
|
9 |
Syzygium samarangense |
leaves |
153.7 |
|
|
10 |
branches |
160.5 |
||
|
11 |
Passiflora edulis |
leaves |
21.2 |
|
|
12 |
branches |
44.3 |
||
|
13 |
Litchi chinensis |
leaves |
48.3 |
|
|
14 |
Malpighia emarginata |
leaves |
155.7 |
|
|
15 |
branches |
38.9 |
||
|
16 |
Averrhoa carambola |
leaves |
131.8 |
|
|
17 |
branches |
0.9 |
||
|
18 |
Acca sellowiana |
leaves |
30.2 |
|
|
19 |
branches |
146.0 |
||
|
20 |
Olea europaea |
leaves |
74.8 |
|
|
21 |
branches |
91.4 |
GAE, gallic acid equivalent
Table 3: Antioxidant, anti-tyrosinase, and anti-elastase activities of Jeju subtropical plant extracts
|
Numbers |
Scientific Names |
Parts |
IC50 (μg/mL) |
|||
|
DPPH |
ABTS |
Tyrosinase |
Elastase |
|||
|
1 |
Sechium edule |
leaves |
- |
371.8 |
- |
- |
|
2 |
Momordica charantia L. |
leaves |
- |
185.4 |
- |
- |
|
3 |
flesh |
- |
273.6 |
- |
- |
|
|
4 |
peel |
- |
452.6 |
- |
- |
|
|
5 |
Carica papaya L. |
leaves |
378.8 |
180.66 |
- |
- |
|
6 |
fruit |
- |
- |
- |
- |
|
|
7 |
Saccharum officinarum |
leaves |
299.5 |
82.88 |
- |
- |
|
8 |
Gynura bicolor |
aerial part |
165.0 |
124.4 |
- |
- |
|
9 |
Syzygium samarangense |
leaves |
36.0 |
10.63 |
284.8 |
385.6 |
|
10 |
branches |
48.0 |
10.36 |
1184.13 |
1211.9 |
|
|
11 |
Passiflora edulis |
leaves |
590.5 |
208.46 |
- |
- |
|
12 |
branches |
110.8 |
30.7 |
- |
- |
|
|
13 |
Litchi chinensis |
leaves |
76.8 |
11.3 |
1335.2 |
– |
|
14 |
Malpighia emarginata |
leaves |
248.0 |
70.5 |
1483.4 |
– |
|
15 |
branches |
69.4 |
70.3 |
1008.0 |
– |
|
|
16 |
Averrhoa carambola |
leaves |
61.3 |
55.3 |
- |
1246.1 |
|
17 |
branches |
71.9 |
53.0 |
- |
1487.8 |
|
|
18 |
Acca sellowiana |
leaves |
46.1 |
10.3 |
1475.7 |
1402.6 |
|
19 |
branches |
7.2 |
43.0 |
550.7 |
992.5 |
|
|
20 |
Olea europaea |
leaves |
101.3 |
44.0 |
- |
1260.4 |
|
21 |
branches |
44.2 |
48.4 |
- |
1079.5 |
|
|
Controls |
Quercetin |
25.5 |
22.5 |
|||
|
Vitamin C |
5.2 |
8.2 |
||||
|
BHT |
22.2 |
23.01 |
||||
|
Arbutin |
86.5 |
|||||
|
Oleanolic acid |
95.0 |
|||||
DPPH, 2,2-diphenyl-1-picrylhydrazyl; ABTS, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid, IC50, half maximal inhibitory concentration
Anti-inflammation capacity
NO overproduction is harmful and results in various chronic inflammatory skin conditions such as atopic dermatitis and acne bulgaris. Therefore, pharmacological blockade of NO production offers promising strategies for therapeutic intervention in skin inflammatory conditions10-12. Consequently, we evaluated the effect of the 21 subtropical plant extracts on NO synthesis in activated macrophages. The Griess reaction, a spectrophotometric method for the determination for nitrite, was used to quantify the nitrite levels (NO production) in the conditioned medium of LPS-treated RAW 264.7 cells treated and Fig. 1 shows the inhibitory activity of the subtropical plant extracts against NO production by these macrophages. Of the 21 test extracts, six showed a greater than 50% inhibition of NO production at a concentration of 500 μg/mL in the culture media. Furthermore, two of the six extracts (M. charantia and S. samarangense, both leaves) showed the most potent, concentration-dependent inhibition with IC50 values of 193.1 and 154.3 μg/mL, respectively (Fig. 1). In addition, Fig. 1 shows that the number of viable activated macrophages was not significantly altered by 20 of the 21 extracts, as determined in the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays, thereby indicating that the inhibition of NO synthesis by the 20 subtropical plant extracts was not due to cytotoxic effects alone.
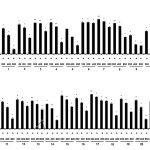 |
Figure 1: Inhibitory effects of subtropical plant extracts on nitric oxide (NO) production in RAW 264.7 cells NO production was assayed in culture medium of lipopolysaccharide (LPS, 1 μg/mL)-stimulated cells (24 h) and treated with extracts (200, 400, and 800 μg/mL). Cytotoxicity was determined using lactate dehydrogenase (LDH) assay. Values are mean ± standard error of the mean (SEM) of triplicate experiments. *P < 0.05 and **P < 0.01. Click here to View figure |
Conclusion
In conclusion, our study demonstrated that S. samarangense leaves efficiently inhibited the LPS-stimulated NO production concentration-dependently, which indicates that this and the other subtropical plants possess several biologically active components that may be potentially useful efficacious inhibitors of the skin aging and inflammation-related processes. Further studies will be focused on using in vitro cell based assays to comprehensively elucidate and chemically identify the major active components mediating the anti-aging and anti-inflammatory effects of these subtropical plants.
References
- Karim, A.A.; Azlan, A.; Ismail, A.; Hashim, P.; Gani, S.S.A.; Zainudin, B.H.; Abdullah, A.A.; BMC Complementary and Alternative Medicine, 2014, 14:381.
CrossRef - Chompoo, J.; Upadhyay, A.; Fukuta, M.; Tawata, S.; BMC Complementary and Alternative Medicine, 2012, 12:106.
CrossRef - Popoola, O.K.; Marnewick, J.L.; Rautenbach, F.; Ameer, F.; Iwuoha, E.I.; Hussein, A.A.; Molecules, 2015, 20, 7143-7155.
CrossRef - Thring, T.S.A.; Hili, P.; Naughton, D.P.; BMC Complementary and Alternative Medicine, 2009, 9, 27.
CrossRef - Ndlovu, G.; Fouche, G.; Tselanyane, M.; Cordier, W.; Steenkamp, V.; BMC Complementary and Alternative Medicine, 2013, 13:304.
CrossRef - Lee, K.K.; Choy, J.J.; Park, E.J.; Choiy, J.D.; International Journal of Cosmetic Science, 2001, 23, 341-346.
CrossRef - Lee S.H.; Sancheti S.; Sancheti, S.; Seo, S.Y.; American Journal of Pharmacology and Toxicology, 2009, 4, 127-129.
CrossRef - Azmi, N.; Hashim, P.; Hashim, D.M.; Halimoon, N; Majid, N.M.N; Asian Pacific Journal of Tropical Biomedicine, 2014, 4(Suppl 1), S348-S352.
CrossRef - Rao, G.V.; Sahoo, M. M.R.; Madhavi, S.L.; Mukhopadhyay, T.; Der Pharmacia Lettre, 2014, 6, 69-73.
- Kim, M.J.; Kim, S.J.; Kim, S.S.; Lee, N.H.; Hyun, C.G.; EXCLI Journal, 2014, 13, 123-136.
- Moon, J.Y.; Yang, E.J.; Kim, S.S.; Kang, J.Y.; Kim, G.O.; Lee, N.H.; Hyun, C.G.; Yakugaku Zasshi, 2011, 131, 961-967.
CrossRef - Yang, E.J.; Moon, J.Y.; Lee, J.S.; Koh, J.; Lee, N.H.; Hyun, C.G.; Journal of Biomedicine and Biotechnology, 2010, 2010, 715739.
- Moon, J.Y.; Yim, E.Y.; Song, G.; Lee, N.H.; Hyun, C.G.; EurAsian Journal of BioSciences, 2010, 4, 41-53.
CrossRef - Yu, Z.; Liu, L.; Xu, Y.; Wang, L.; Teng, X.; Li, X.; Dai, J.; Carbohydrate Polymers, 2015, 132, 180-186.
CrossRef - Zhu, K.X.; Lian, C.X.; Guo, X.N.; Peng, W.; Zhou, H.M.; Food Chemistry, 2011, 126, 1122-1126.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









