GC-MS/MS Based Identification of Bioactive principles of Chloroform fraction of Swertia Chirayatia (Chirata).
Homa Jilani Khan1,2, Mohammad Kaleem Ahmad2, Abdul Rahman Khan1, Namrata Rastogi2, Jamal Akhtar Ansari1,2, Nishat Fatima1,2,, G. N. V. Satyanarayan3, and Abbas Ali Mahdi2*
1Department of Chemistry, Integral University, Lucknow, 226 026 India.
2Natural Products Research Lab, Department of Biochemistry, King George’s Medical University, Lucknow,226 00, India.
3Analytical Chemistry, CSIR-Indian Institute of Toxicological Research, Lucknow, 226003 India.
Corresponding author email: abbasalimahdi@kgmcindia.edu
DOI : http://dx.doi.org/10.13005/ojc/320213
Article Received on :
Article Accepted on :
Article Published : 25 Apr 2016
The aim of this study is to characterize the chemical composition, in vitro antioxidant and anticancer activity of chloroform fraction of Swertia chirata L. (Family Gentianaceae). GC-MS analysis of chloroform fraction of S. chirata (CFSc) identified twenty-five compounds belonging to different chemical class. Among these important classes of compounds were tri-terpenoids, phenol, acids, and fatty acids. Our phytochemical analysis of CFSc was well supported by its Total Phenolic Content and antioxidant activity with in vitro anticancer activity which was evaluated by MTT assay also showed that CFSc exhibited anti-proliferative activity against human breast cancer cell lines (MDA-MB-231 and MCF-7). In conclusion; our study revealed an extensive biochemical profile of CFSc as well its potential as a pharmacological agent with respect of its antioxidant and anti-cancer property. More safety and toxicological studies are needed to be addressed to ensure the use of CFSc for medicinal purposes.
KEYWORDS:GC-MS; Tri-terpenoids; Phenols; MTT assay; Anti-proliferative
Download this article as:| Copy the following to cite this article: Khan H. J, Ahmad M. K, Khan A. R, Rastogi N, Ansari J. A, Fatima N, Satyanarayan G. N. V, Mahdi A. A. GC-MS/MS Based Identification of Bioactive principles of Chloroform fraction of Swertia Chirayatia (Chirata). Orient J Chem 2016;32(2) |
| Copy the following to cite this URL: Khan H. J, Ahmad M. K, Khan A. R, Rastogi N, Ansari J. A, Fatima N, Satyanarayan G. N. V, Mahdi A. A. GC-MS/MS Based Identification of Bioactive principles of Chloroform fraction of Swertia Chirayatia (Chirata). Orient J Chem 2016;32(2). Available from: http://www.orientjchem.org/?p=15525 |
Introduction
Since ancient times plants parts and their products have been used for their innate medicinal properties and the search is still ongoing. Many plant based natural compounds have shown their efficacy as anti-microbial, anti-cancer, anti-viral as well as anti-inflammatory agents 1. Moreover, plants have yielded many potent drugs that are very much in use in modern medicine and morphine is one such drug which is a known natural analgesic, 2 and was isolated from opium poppy (latex of Papaver somniferum fruit). Medicinal plants are potential source of secondary metabolites in the form of flavonoids, anthocyanins, tannins, dietary glutathionine, and vitamins and these metabolites are the actual determinants of their pharmacological properties 3. Detailed biochemical analysis of these metabolites have identified agents like Taxol 4, from Taxus brevifolia, Vincristine and Vinblastine 5 from Vinca rosea Linn.(Periwink Plant), all of them are important anticancer drugs. These beneficial phytochemicals either act by inhibiting of the production of free radicals and/or inactivating free radicals by quenching singlet and triplet oxygen, decomposing hydrogen peroxide and inhibiting various enzymes 3. For example, phenolic compounds in medicinal plants possess strong antioxidant activity and hence protect cells against the oxidative damage caused by free radicals 6, 7. Since free radicals are proven core for igniting pathogenesis of various serious diseases e.g. cancer, atherosclerosis and neurodegenerative diseases 8, therefore, emphasis should be made on inhibition of free radicals either for preventive or for curative measures for such diseases and use of natural agents can be a plausible choice in this regard.
For India and its neighboring countries, Swertia chirata of family Gentianaceae is an esteemed herb because of its wide and easier availability. S. chirata plant is generally known to possess anti-viral (9), anti-inflammatory (10), anthelmintic (11), and anti-carcinogenic (12) activities due to the presence of phytochemicals like flavonoids, xanthones, terpenoids, iridoid , amarogentin, swerchirin, opelic acid and gentiopicriseco iridoid glycosides (13). However, identification of the biochemical entities of chloroform fraction of S. chirata (CFSc) has not been previously explored.
Table 1: Summary of compounds found in chloroform fraction of S. chirata.
| S.No | Compounds name (NIST listed Library) | R.T | M.W | Relative peak area (%) | M.F |
| 1. | Xanthone[TMS] | 7.13 | 196 | 0.07 | C13H8O2 |
| 2. | Succinic acid,[TMS] | 7.41 | 438 | 0.04 | C16H38O6Si4 |
| 3. | β-D-Galactopyranoside,methyl 2,3-bis-O-(trimethylsilyl)-, cyclic butylboronate | 10.61 | 404 | 0.22 | C17H37BO6Si2 |
| 4. | Acetaldehyde,[(trimethylsilyl)oxy],O-[bis[(trimethylsilyl)oxy]phosphophinyl]oxime | 12.63 | 371 | 0.63 | C11H30NO5PSi3 |
| 5 | 1-Trimethylsiloxy-2-(1-cyclohexenyl)ethane | 16.77 | 198 | 0.61 | C11H22OSi |
| 6. | O,N-Permythylated N-Acetyllsine | 18.16 | 272 | 1.38 | C13H24N2O4 |
| 7. | 1-Ethyl-1-heptanoyloxy-1-silacyclopentane | 19.34 | 242 | 1.16 | C13H26O2Si |
| 8. | 1-Cyclohexanone,5-[2{[1-[1,1-dimethylethy]-1,1-dimethylsilyl]oxy}-1-methylethyl]-2-methyl | 20.69 | 284 | 1.27 | C16H22O2Si |
| 9. | β-D-Galactopyranoside, methyl2,3-bis-O-[timethysilyl]-,cyclic methylboronate | 21.22 | 362 | 0.72 | C14H31BO6Si2 |
| 10. | Benzoic acid-3-methoxy-4-[(trimethylsilyl)oxy] | 22.45 | 312 | 0.72 | C14H24O4Si2 |
| 11. | Azelaic acid,bis(trimethylsilyl) ester | 23.05 | 332 | 0.17 | C15H32O4Si2 |
| 12. | Silane, trimethyl{[1-(1-methyl-1-butenyl)cyclohexyl}oxy) | 24.6 | 240 | 1.33 | C14H48OSi |
| 13. | Hexadecanoic acid, trimethylsilyl ester | 25.20 | 270 | 1.57 | C19H40O2Si |
| 14. | Hexadecanoic acid, methyl ester | 27.27 | 328 | 5.09 | C17H34O2 |
| 15. | Cis-13-Octadecenoic acid, methyl ester | 28.03 | 296 | 2.56 | C19H36O2 |
| 16. | 9,12-Octadecadienoic acid(Z,Z),trimethylsilyl ester | 29.65 | 352 | 0.43 | C21H40O2Si |
| 17. | trans-9-Octadecenoic acid, trimethylsilyl ester | 29.74 | 354 | 0.94 | C21H42O2Si |
| 18. | Octadecanoic acid, trimethylsilyl ester | 30.11 | 356 | 3.4 | C21H44O2Si |
| 19. | Hexadecanoic acid,2-{[trimethylsilyl]oxy}-1-[[(trimethylsilyl)oxy]methyl]ethyl ester | 33.74 | 474 | 2.75 | C25H54O4Si2 |
| 20. | Docosanoic acid, trimethylsilyl ester | 34.23 | 412 | 2.15 | C25H52O2Si |
| 21. | Bis[trimethylsilyl)monostearin | 35.36 | 502 | 1.62 | C27H58O4Si2 |
| 22. | Tetracosanoic acid, trimethylsilyl ester | 35.82 | 440 | 1.23 | C27H56O2Si |
| 23. | Viminalol | 42.01 | 426 | 3.76 | C30H50O |
| 24. | Lup-20[29]-en-3-one | 42.90 | 424 | 2.1 | C30H48O |
| 25. | Urs-12-en-28-al,3-[acetyloxy] | 47.25 | 482 | 13.88 | C32H50O3 |
Therefore in present study, we for the first time carried out the entire biochemical profile of CFSc by GC-MS/MS method. We also identified the phytochemical components of this fraction which are probably responsible for antiproliferative activity against human breast cancer cell lines (MDA-MB-231 and MCF-7) as observed in this study.
Materials and Methods
Collection and identification of plant material
The adequate quantity of aerial part of S. chirata was purchased and identification was confirmed by Department of Botany, Shia P.G. College, Lucknow. After washing and, drying the plant material was ground to fine powder form. This was stored in a clean, dry and sterile container for further use.
Preparation of Extract
The powdered material (500 g) was extracted in methanol (100%) by percolation at room temperature. The extract was concentrated in vacuum evaporator, and was kept in vacuum desiccators for complete removal of solvent and weighed (quantity≈70g). This crude methanolic extract was further utilised for preparation of chloroform fraction by cold maceration process (fig 1). Vacuum evaporator was used to concentrate chloroform fraction and then it was kept in vacuum desiccator for complete removal of solvent leaving the weighed (quantity≈10g) chloroform fraction. The percentage yield was expressed in terms of air dried weight of plant material.
Table 2: IC50 Value Obtained For Cfsc Against MCF-7 And MDA-MB-231 Cancer Cells After Exposure Of 24h, 48h And 72h. Values Are Expressed As Mean ± S.D. (N = 3).
| MCF-7 IC 50 (µg/ml) | |||
| Time | 24 h | 48 h | 72 h |
| CFSc | 50.8±1.3 | 39.2±2.3 | 17.5±0.5 |
| MDA-MB-231 IC 50 (µg/ml) | |||
| Time | 24 h | 48 h | 72 h |
| CFSc | 62.5±1.1 | 49.2±2.7 | 34.2±1.5 |
GC-MS/MS Analysis
GC-MS/MS of Thermo TSQ Quantum XLS with Triplus autosampler equipped with triple quadrapole was used for the identification of different components of chloroform fraction. The sample was injected on Elite-5MS (30 m x 0.25 mm ID, 0.25 µm film thickness) column. Helium gas (99.999%) was used as carrier gas at a constant flow rate of 1.1ml/min. with splitless mode, and sample (1µl) was injected into the system. The injector port temperature was maintained at 250°C, the mass transfer line temperature was maintained at 300°C, the oven temperature was programmed at 65°c(hold for 2 min), with an increase of 6° c/min. to 230°C, then 10°C/min to 290°C, ending with a 20 min. hold. The solvent delay was 6.5 min. and the total GC-MS running time was 55 min. with full scan mode with injector port silylation in which derivatizing agent was used BSTFA (1.5µl).
Various compounds were identified by their retention time and based on NIST MS 2.0 library research has derived mass fragmentation pattern of the CFSc (Fig 2.).
Determination of the total phenolic content
Total phenol contents were determined by Folin-Ciocalteu reagent method with slight modifications (14) using Gallic acid as a standard phenolic curve. In brief 0.1 ml of CFSc (1mg/ml) was added to 1 ml 10% Folin–Ciocalteu reagent and incubates for five minute. After five minute 0.8 ml solution of Na2CO3 (10% w/v in distilled water) was added and allowed to stand for 15 minutes at 250C with alternating shaking. Sample was vortexed again and the absorbance of resultant solutions was measured at 725 nm using Thermo-Fisher spectrophotometer after incubation at 25 ºC for 1 h. The total phenolic contents were expressed as mg of Gallic acid equivalent (GAE)/ g plant dried weight.
Free radical-scavenging activity: DPPH assay
DPPH (1, 1-diphenyl-2-picrylhydrazyl) radical scavenging activity was evaluated based on method (15). A stock solution of 0.052 mg/ml of DPPH was prepared in methanol. The diluted fraction (1mg/ml) was mixed with DPPH solution, concentrations range (6.25 – 400 µg/ml) and allowed to stand in dark at RT for 20 minutes. The control was prepared as above without any extract. The reaction mixture was observed at 517nm against a water blank, with a Double Beam Spectrophotometer 2203 using Ascorbic acid as reference material. DPPH radical scavenging activity was calculated as follows:
% inhibition = [Abs sample – Abs control)/ (Abs control)] X 100
Control = 1.0 mL methanol + 1.0 mL DPPH
Cell line and culture
Human breast cancer (MDA-MB231 and MCF-7) cell line was obtained from Cell repository, National Center for Cell Science (NCCS), Pune, India. MDA-MB-231 and MCF-7 were cultured with medium RPMI supplemented with 5% FBS and 10% FBS respectively with 1% penicillin/ streptomycin solution and grown at 37ºC, 5% CO2 in a humidified chamber.
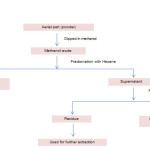 |
Figure 1: Flow chart depicting processing of S. chirata to produce chloroform fraction. Click here to View figure |
Cell Viability assay
MTT reduction assay was used to evaluate anti-proliferative activity of CFSc. This assay is based on enzymatic reduction phenomenon of MTT (3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) dye. The assay provides a direct relationship between the viable cells and color formation (absorbance). For this purpose, breast cancer cells (5×103) were seeded in 100 μL RPMI media in each well of a 96-well culture plate for 24h at temperature of 37oC and humidity of 5% CO2. Stocks of sample were prepared in DMSO and diluted to desired concentrations (6.25 to 400µg/ml) in media and added to wells in triplicate manner. After 24h of treatment, 10 μL of MTT (5 mg/mL) dye was added to each well, and then it was further incubated for ≈1h at 37oC until development of formazan blue crystals. The supernatant was discarded from each well, and then 100μL of DMSO was added to solubilize formazan crystals for 10 min. at 37oC. The absorbance was recorded at 540 nm by a microplate reader (BIORAD-680). The percentage viability was calculated by using the formula:
% Growth inhibition = 100 – %Cell viability*
Where *% Cell viability = [OD of treated)/ (OD of control)] X 100
(OD = optical density)
Statistical Analysis
The data presented as means SD were analyzed using ANOVA. Dunnett’s multiple range test (DMRT) was used to determine significant differences between means. The results were considered statistically significant if the P values were 0.05 or less.
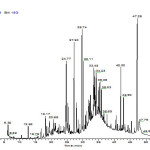 |
Figure 2: GC-MS/MS chromatogram of CFSc. Click here to View figure |
Results and Discussion
Phytochemical contents of Chloroform Fraction of S. chirata
GC-MS/MS based qualitative and quantitative analysis of CFSc revealed presence of different volatile organic compounds listed in Table 2 with distinctive chromatogram as shown in Fig. 2. The CFSc was analyzed through GC-MS and it was found to contain 25 chemical constituents eluted between 7.13 and 47.25 min. From the qualitative and quantitative analysis of the CFSc, these 25 compounds were tentatively classified into different chemical classes including acid , alcohol , aldehyde, ester, hydrocarbon, ketone, fatty acids and some other compounds which were grouped as miscellaneous. The major phytochemicals identified in CFSc were xanthones, terpenoids, and polyphenols. These findings are well in accordance with previous reports where ethanolic extracts of S. chirata have shown the presence of these phytochemicals in significant amounts (16, 20). Among these phytochemicals the anti-tumor activity of xanthones is well known, which include cell cycle arrest, suppression of tumor cell proliferation, induction of apoptosis and differentiation, reduction of inflammation, and inhibition of adhesion, invasion, and metastasis (21-23). Presence of this important phytochemical in CFSc entails its significant medicinal property. Another chemical class which was found to be present in CFSc was fatty acids such as docosanoic acid, benzoic acid and azalic acid. These fatty acids have also been shown to exhibit pharmacological properties such as anti-inflammatory, anti-cancer and anti-diabetic 24, 25. Furthermore, our findings also revealed tri-terpenoids as the most dominant chemical class found in CFSc with highest proportion (13.88%). Interestingly we found two major bioactive compounds belonging to tri-terpenoids family, [(Urs-12-en-28-al,3-acetyloxy)] which is Ursolic acid derivative and viminalol to be present in CFSc in substantial amounts. The presences of these tri-terpenes have not been previously reported in CFSc. However, both Ursolic acid is nontoxic, penta-cyclic tri-terpenoid and viminalol (α-amyrin) are known for their widespread medicinal properties such as anti-inflammatory, chemo-protective, antimicrobial, antinociceptive and hepatoprotective and antioxidant activities 17, 18,19. Our GC-MS/MS analysis results indicated the presence of many bioactive principles in CFSc which may also have significant effect on its medicinal properties.
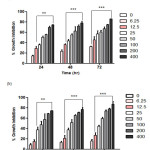 |
Figure 3: Anti-proliferative effect of CFSc against human breast cancer cells. Human breast cancer MCF-7(a) and MDA-MB-231(b) cells were exposed for concentrations range (6.25µg/ml – 400µg/ml) for 24, 48 and 72h, and the viability of the cells was determined by MTT assay. Cell viabilities are shown as percentages, and the untreated cells were regarded as 100% viable. Data represent the means of three experiments conducted in triplicate and were significant (P≤0.05). Click here to View figure |
Total phenolic content of Chloroform fraction of S. chirata
Among many secondary metabolites of plants, the phenolic or polyphenols are very important by virtue of their antioxidant activities through chelating redox-active metal ions, inactivating lipid free radical chains, and avoiding the hydro-peroxide conversions into reactive oxy-radicals (26). Most of the plant based anti-cancer compounds belong to the polyphenols sub-family of natural products and contribute to their anti-proliferative activity (27). It is expressed in terms of Gallic acid equivalent (the standard curve equation: y = 7.026x – 0.0191, r2 = 0.999). In our study, we reported the total phenolic content of the CFSc which was found to be 9.1mg, which is expressed as mg of GAE/g of extract. Therefore, our results suggested that CFSc contained high overall levels of biochemically active polyphenols.
Total phenolic content of Chloroform fraction of S. chirata
Among many secondary metabolites of plants, the phenolic or polyphenols are very important by virtue of their antioxidant activities through chelating redox-active metal ions, inactivating lipid free radical chains, and avoiding the hydro-peroxide conversions into reactive oxy-radicals (26). Most of the plant based anti-cancer compounds belong to the polyphenols sub-family of natural products and contribute to their anti-proliferative activity (27). It is expressed in terms of Gallic acid equivalent (the standard curve equation: y = 7.026x – 0.0191, r2 = 0.999). In our study, we reported the total phenolic content of the CFSc which was found to be 9.1mg, which is expressed as mg of GAE/g of extract. Therefore, our results suggested that CFSc contained high overall levels of biochemically active polyphenols.
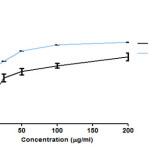 |
Figure 4: Free radical scavenging effect of CFSc in DPPH assay Click here to View figure |
Free radical scavenging activity of Chloroform fraction of S. chirata by DPPH
The DPPH test determines the antioxidant activity based on the ability of the phenols to neutralize the stable free radical DPPH (28).In order to determine the free radical scavenging activity of CFSc, we performed DPPH assay in a dose-dependent scavenging activity of free radicals as shown in (Fig 2). The highest free radical scavenging activity (77.5%) was observed at 200µg/ml with an IC50 of 23±3.2μg/ml and this activity was comparable to that of ascorbic acid (6±8.2µg/ml). Free radical scavenging activity has been significantly associated with the property of natural compounds to inhibit the deleterious effects of free radicals. Protection of cells from free radical can reduce chances of initiation of diseases like cancer (30). Therefore, these results suggested that CFSc possessed significant free radical scavenging activity and so can be developed as chemo-preventive agent against cancer.
Growth inhibition of breast cancer cells by Chloroform fraction of S. chirata
In present study, anti-proliferative activity of CFSc against two preselected human breast cancer cells MCF-7 and MDA-MB-231 was assayed by using MTT dye. MDA-MB-231 and MCF-7 cancer cells were treated with different concentrations of CFSc ranging from 6.25µg/mL-400µg/mL, and the bar graph indicated percentage inhibition in treated cells verses untreated cells. The IC50 as compared to that of untreated cells was determined as shown in (Table-1). The CFSc demonstrated potent anti-proliferative activity against human breast cancer cells (MCF-7 and MDA-MB-231) in a dose and time-dependent manner (Fig 2).
The anti-cancerous activity of medicinal plants is attributed to their phytochemical content (31, 32). This is evidenced by previous reports which have indicated a direct association between the amount of polyphenols, tri-terpenoids present in medicinal plants and their cancer prevention ability (30, 33). Hence, from our experimental findings it could be concluded that presence of adequate amount of polyphenols, Fatty acids, tri-terpenoids, may be responsible for anti-cancer activity of CFSc.
Conclusion
The GC-MS analysis of the volatile compounds of CFSc showed the presence of variety of compounds in which major groups were tri-terpenoids, fatty acids and, ketones group. However the major highlight of our study is the identification of two important bioactive components [(Urs-12-en-28-al,3-acetyloxy)] and viminalol in CFSc. The presence of these two along with other phytochemicals can be attributed to the significant antioxidant as well as anti-proliferative activity of CFSc against human breast cancer cells. However, ongoing studies in our laboratory are focusing on isolation of active compounds from CFSc in search of its potent chemo-preventive agent. Future studies are warranted for validation of our results through using herbal preparation of CFSc as complementary and alternative medicine for breast cancer.
Acknowledgement
The author is very grateful to UGC/MANF (F1-17.1/2012-13/MANF-2012-13-MUS-UTT-15179) for providing fund and Department of Biochemistry, King George Medical University, Luknow, for supporting this work.
References
- Si-Yuan Pan.; Shu-Feng, Zhou.; Si-Hua, Gao.; Zhi-Ling, Yu.; Shuo-Feng, Zhang.; Min-Ke, Tang.; Jian-Ning, Sun.; Dik-Lung, Ma.; Yi-Fan, Han.; Wang-Fun, Fong.; Kam-Ming, Ko. Evid. Based Complement Alternat.Med. 2013, ,627375.
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Mar. Drugs. 2014, 12, 1066–1101.
CrossRef - Pandey, K. B. & Rizvi, S.I. Oxid. Med.Cell. Longev. 2009, 2, 270–278.
CrossRef - Dias, D. A.; Urban, S.; Roessner, U. Metabolites. 2012, 2, 303–336.
CrossRef - William, B.P.; Raymond, W.R. Oxford University Press, New York. 1979, 221-232.
- Kahkonen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja,K.; Kujala,T.S.; Heinonen, M. Journal of Agricultural & Food Chemistry, 1999,47,3954–3962.
CrossRef - Kaur, P.; Singh, B.; Kumar, S.; Kaur, S. Journal of Chinese Clinical Medicine, 2008, 3, 278-284.
- Chen, X.; Guo, C.; Kong, J., Neural. Regen. Res. 2012, 7, 376–385.
- Verma, H.; Patil, P.R.; Kolhapure, R.M. Ind. J. Med. Microbiol, 2008, 26, 322-326.
- Iqbal, Z.; Lateef, M.; Khan, M.K.; Jabbar,A.; Akhtar, M.S. Fitotherapia, 2006,77, 463-465.
CrossRef - Islam, C.N.; Banerjee, M.K.; Das, P.C. Indian J. Pharmacol, 1995, 27, 37–39.
- Saha, P.; Mandal, S.; Das, A.; Das, P.C.; Das, S. Phytother. Res. 2004, 18, 373-8.
CrossRef - Latif, A.; Rehman, S. Pharmacophore, 2014, 5,98-108.
- Yu, L.; Haley, S.; Perret, J.; Harris, M.; Wilson, J.; Quian, M. Journal of Agricultural and Food Chemistry, 2002, 50, 1619–1624.
CrossRef - Blois, M.S. Nature, 1958, 26,1199-1200.
CrossRef - Chatterjee,A.; Pakrashi, S.C. “The Treatise on Indian Medicinal Plants used in Ayurveda” Publication and Information Directorate, New Delhi: India, 1995 4,. 92.
- Rastogi, R. P.; & Mehrotra, B.,N. “Compendium of Indian medicinal plants” CDRI, Lukhnow and National institute of Science Communication, New Delhi: India Vol. 2(1991), p. 654; Vol. 3(1993), p. 615;Vol. 4(1995), p. 701; Vol. 5(1998), pp. 815.
- Melo, C.M.; Bezerra, M.K.C.; Neves, C.S.J.; Morais, T.C.; Rao, S.V.; Santos, F.A.; Brito, G.A.; Chaves, M.H. World Journal of Gastroenterol, 2010, 16, 4272–4280.
CrossRef - Oliveira, F.A.; Chaves, M.H.; Almeida, F.R.; Lima, R.C.; Silva, R.M.; Maia, J.L.; Brito, G.; Santos, F.A.; Rao, V. Journal of Ethnopharmacology,2005,98,103-8.
CrossRef - Bhattacharya, S.K.; Reddy, P.K.S.P.; Ghosal, S.; Singh, A.K.; Sharma, P.V. J. Pharm. Sci., 1976, 65, 1547-1549.
CrossRef - Akao, Y.; Nakagawa, Y.; Iinuma, M.; Nozawa,Y. Int. J. Mol. Sci. 2008, 9, 355–70.
CrossRef - Pedraza-Chaverri, J.; Cardenas-Rodriguez, N.; Orozco-Ibarra, M.; Pérez-Rojas, J.M. Food. Chem .Toxicol. 2008,46, 3227–39.
CrossRef - Hung, S.H.; Shen, K.H.; Wu, C.H.; Liu, C.L.; Shih, Y. W. J. Agr. Food Chem. 2009, 57, 1291–8.
CrossRef - Jing K.; Wu T.; Lim K. Anticancer Agents Med. Chem, 2013, 13, 1162-77
CrossRef - Das, U.N. J. Assoc. Physicians India. 2006, 54, 309-19
- Esmaeili, A. K..; Taha, R. M.; Mohajer, S.; Banisalam, B. BioMed Research International. 2015, 2015 Article ID 643285, 11
- Gülçin, I. Life Sci. 2006, 78, 803-811.
CrossRef - Hseu, Y.; Chang, W.; Chen, C.; Liao, J.; Huang, C.; Lu, F.; Chia, Y.; Hsu, H.; Wu, J.; Yang, H. Food and Chemical Toxicology, 2008, 46, 105–114,
CrossRef - Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Food Chem. Toxicol., 2008, 46, 446- 475.
CrossRef - Adorjan, B.; Buchbauer, G. Flavour Fragr. J., 2010, 25, 407-426.
CrossRef - Baser, H.C.; Buchbauer, G. Handbook of essential oils science, technology, and applications. CRC Press Taylor & Francis Group, New York, United States of America,2010.
- La V.C.; Altieri A.; Tavani A. Vegetables, fruit, antioxidants and cancer: a review of Italian studies. Eur.J. Nutr. 2001, 40, 261–7.
CrossRef - Terry, P.; Terry, J.B.; Wolk, A. J. Intern. Med. 2001,250, 280–90.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









