Antibacterial effect of ginger (Zingiber officinale) roscoe and bioactive chemical analysis using Gas chromatography mass spectrum
Hasanain Khaleel Shareef 1, Haidar J. Muhammed2 , Haider Mashkoor Hussein3 and Imad Hadi Hameed4
1Department of Biology, Babylon University, Iraq
2College of Science, Almustansriya University, Iraq
3College of Science, Al-Qadisiya University, Iraq
4Department of medical science, Babylon University, Iraq.
Corresponding author email: imad_dna@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/320207
Article Received on :
Article Accepted on :
Article Published : 05 May 2016
The objectives of this research was to determine the chemical composition of roscoe extract from methanol and evaluation of antibacterial activity. The phytochemical compound screened by GC-MS method. Forty eight bioactive phytochemical compounds were identified in the methanolic extract of Zingiber officinale. The identification of phytochemical compounds is based on the peak area, retention time molecular weight, molecular formula, MS Fragment-ions and pharmacological actions. GC-MS analysis of Zingiber officinale revealed the existence of the Octanal, 2-Naphthalenamine,1,2,4a,5,6,7,8,8a-octahydro-4a-methyl, 1-(Cyclopropyl-nitro-methyl)-cyclopentanol, Endo-Borneol, Decanal, 1,2-15,16-Diepoxyhexadecane, Propanal,2-methyl-3-phenyl, Benzeneacetic acid ,4-(1H-1,2,3,4-tetrazol-1-yl), Ascaridole epoxide, 2-Methoxy-4-vinylphenol, 6-epi-shyobunol, Phenol,2-methoxy-5-(1-propenyl)-,E, Alfa.-Copaene, 8-Isopropenyl-1,5-dimethyl-cyclodeca-1,5-diene, Bicyclo[3.1.0]hexane-6-methanol,2-hydroxy-1,4,4-trimethyl, 7-epi-cis-sesquisabinene hydrate, Alloaromadendrene, Benzene,1-(1,5-dimethyl-4-hexenyl)-4-methyl, 1,3-Cyclohexadiene ,5-(1,5-dimethyl-4-hexenyl)-2methyl-,[S-(R*,S*)], Aromadendrene oxide, 1,6,10-Dodecatrien-3-ol,3,7,11-trimethyl-,(E), 4-((1H)-3-Hydroxy-1-propenyl)-2-methoxyphenol, Butan-2-one,4-(3-hydroxy-2-methoxyphenyl), Longipinocarveol,trans, Cholestan-3-ol,2-methylene-,(3ß,5α)-, Bicyclo[4.4.0]dec-2-ene-4-ol,2-methyl-9-(prop-1-en-3-ol-2-yl)-, Corymbolone, Estra-1,3,5(10)-trien-17ß-ol, 1-Heptatriacotanol, Fenretinide, Folic acid, Spiro[4.5]decan-7-one,1,8-dimethyl-8,9-epoxy-4-isopropyl-, 7H-6,9a-Methano-4H-cyclopenta[9,10] cyclopropa[5,6]cyclodeca[1, Gingerol, 1b,4a-Epoxy-2H-cyclopenta[3,4]cyclopropa [8,9]cycloundec[1,2-b]o, Cyclopropa[5,6]-A-nor-5α-androstane-3,7-dione,3´,6ß-dihydro-17ß-h, Olean-12-ene-3,15,16,21,22,28-hexol,(3ß,15α,16α,21ß,22α)-, Benz[e]azulen-3(3aH)-one,4,6a,7,8,9,10,10a,10b-octahydro-3a,8,1, Naphthalene, decahydro-1-pentadecyl-, 13-Docosenamide,(Z)-, 9,10-Secocholesta-5,7,10(19)-triene-3,24,25-triol, (3ß,5Z,7E)-, n-(2,4-Dinitrophenyl)-N´-13-(2,6,6-trimethyl-cyclohex-1-enyl)propylider, n-(2,4-Dinitrophenyl)-N´-13-(2,6,6-trimethyl-cyclohex-1-enyl)propylider, Ingol 12-acetate, 2,2,4-Trimethyl-3-(3,8,12,16-tetramethyl-heptadeca-3,7,11,15-tetrae, Piperine, 2-Methylcortisol, 9-Desoxo-9-x-acetoxy-3,8,12,-tri-O-acetylingol and Propanoic acid ,2-(3-acetoxy-4,4,14-trimethylandrost-8-en-17-yl. Methanolic extract of bioactive compounds of Zingiber officinale was assayed for in vitro antibacterial activity against Proteus mirabilis,Escherichia coli, Pseudomonas aerogenosa, Proteus mirabilis, Staphylococcus aureus and Klebsiella pneumonia by using the diffusion method in agar. The zone of inhibition were compared with different standard antibiotics. The diameters of inhibition zones ranged from 4.93±0.290 to 0.89±0.210 mm for all treatments.
KEYWORDS:Antibacterial; GC/MS; Bioactive compounds; Zingiber officinale
Download this article as:| Copy the following to cite this article: Shareef H. K, Muhammed H. J, Hussein H. M, Hameed I. H. Antibacterial effect of ginger (Zingiber officinale) roscoe and bioactive chemical analysis using Gas chromatography mass spectrum. Orient J Chem 2016;32(2) |
| Copy the following to cite this URL: Shareef H. K, Muhammed H. J, Hussein H. M, Hameed I. H. Antibacterial effect of ginger (Zingiber officinale) roscoe and bioactive chemical analysis using Gas chromatography mass spectrum. Orient J Chem 2016;32(2). Available from: http://www.orientjchem.org/?p=15782 |
Introduction
Ginger (Zingiber officinale Roscoe, fam. Zingiberaceae) is a perennial herb, slender perennial plant that reaches the height of two feet and has greenish yellow flowers resembling orchids. The rhizome is horizontal, branched, fleshy, aromatic, white or yellowish to brown. Leaves are narrowly or linear-lanceolate, up to 20 cm long and 1.5-2 cm wide. The dried rhizome of ginger contains approximately 1-4% of volatile oils which are the medicinally active constituents and are also responsible for the characteristic odour and taste. Flowers are produced in a dense spike, yellow green with purple endings. This plant is widely distributed in South-Eastern Asia1. It has a long history of medicinal use dating back 2500 years in China and India for conditions such as nausea and vomiting, diarrhea, dyspepsia, rheumatism, and colds2. Other pharmacological actions of ginger and compounds isolated from it include anti-inflammatory, antioxidant3 ; hypoglycemic4 ; analgesic, antiplatelet5, antiemetic6,7, antithrombotic, anti-tumorigenic, radio protective, antimicrobial, antifungal actions8,9. The major pungent compounds in ginger include potentially active gingerols, which can be converted to shogaols, zingerone, and paradol. 6-gingerol appears to be responsible for characteristic taste of ginger and together with 6-shogaol have been shown to have antipyretic, analgesic, anti-inflammatory, anti-tussive and hypotenssive effects10,11. Patients with chronic and painful diseases often seek alternative therapy, and currently ginger is one of the most popular herbal medications for inflammatory diseases12. In food industry, both pathogenic and food spoilage bacteria can attach and form a biofilm on food contact surfaces and food product, on the other hand Z. officinale is widely used as spice, so the aim of this study was ginger effectiveness in preventing this problem through the evaluation of antibacterial activity of methanolic extract of Z. officinale.
Materials and Methods
Collection and preparation of plant material
The roscoe were dried at room temperature for ten days and when properly dried then powdered using clean pestle and mortar, and the powdered plant was size reduced with a sieve. The fine powder was then packed in airtight container to avoid the effect of humidity and then stored at room temperature13,14.
Preparation of sample
About seven grams of the plant sample powdered were soaked in 80 ml methanol individually. It was left for 72 hours so that alkaloids, flavonoids and other constituents if present will get dissolved. The methanol extract was filtered using Whatman No.1 filter paper and the residue was removed15,16.
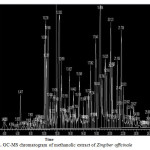 |
Figure 1: GC-MS chromatogram of methanolic extract of Zingiber officinale Cick here to View figure |
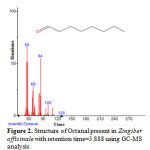 |
Figure 2: Structure of Octanal present in Zingiber officinale with retention time=3.888 using GC-MS analysis. |
Gas chromatography – Mass Spectrum analysis
The GC-MS analysis of the plant extract was made in a (789 Agilent) instrument under computer control at 70 eV. About 1μL of the methanol extract was injected into the GC-MS using a micro syringe and the scanning was done for 45 minutes17,18. As the compounds were separated, they eluted from the column and entered a detector which was capable of creating an electronic signal whenever a compound was detected. The greater the concentration in the sample, bigger was the signal obtained which was then processed by a computer. The time from when the injection was made (Initial time) to when elution occurred is referred to as the retention time (RT).While the instrument was run, the computer generated a graph from the signal called Chromatogram. Each of the peaks in the chromatogram represented the signal created when a compound eluted from the Gas chromatography column into the detector19,20. The x-axis showed the RT and the y-axis measured the intensity of the signal to quantify the component in the sample injected. As individual compounds eluted from the gas chromatographic column, they entered the electron ionization (mass spectroscopy) detector, where they were bombarded with a stream of electrons causing them to break apart into fragments. The fragments obtained were actually charged ions with a certain mass21. The M/Z (mass / charge) ratio obtained was calibrated from the graph obtained, which was called as the Mass spectrum graph which is the fingerprint of a molecule. Before analyzing the extract using gas Chromatography and Mass Spectroscopy, the temperature of the oven, the flow rate of the gas used and the electron gun were programmed initially. The temperature of the oven was maintained at 100°C. Helium gas was used as a carrier as well as an eluent. The flow rate of helium was set to 1ml per minute. The electron gun of mass detector liberated electrons having energy of about 70eV.The column employed here for the separation of components was Elite 1(100% dimethyl poly siloxane). The identity of the components in the extracts was assigned by the comparison of their retention indices and mass spectra fragmentation patterns with those stored on the computer library and also with published literatures. Compounds were identified by comparing their spectra to those of the Wiley and NIST/EPA/NIH mass spectral libraries22.
Determination of antibacterial activity of crude bioactive compounds of Zingiber officinale.
The test pathogens (Klebsiella pneumoniae, Pseudomonas aeruginosa, Klebsiella pneumoniae, E. coli, and Staphylococcus aureus) were swabbed in Muller-Hinton agar plates. 60μl of plant extract was loaded on the bored wells. The wells were bored in 0.5 cm in diameter24. The plates were incubated at 37C° for 24 hrs and examined. After the incubation the diameter of inhibition zones around the discs was measured.
Results and Discussion
Gas chromatography and mass spectroscopy analysis of compounds was carried out in methanolic roscoe extract of Zingiber officinale, shown in Table 1. The GC-MS chromatogram of the 48 peaks of the compounds detected was shown in Figure 1. Chromatogram GC-MS analysis of the methanol extract of Zingiber officinale showed the presence of forty eight major peaks and the components corresponding to the peaks were determined as follows. The First set up peak were determined to be Octanal Figure 2. The second peak indicated to be, 2-Naphthalenamine,1,2,4a,5,6,7,8,8a-octahydro-4a-methyl, Figure 3. The next peaks considered to be 1-(Cyclopropyl-nitro-methyl)-cyclopentanol, Endo-Borneol, Decanal, 1,2-15,16-Diepoxyhexadecane, Propanal,2-methyl-3-phenyl, Benzeneacetic acid ,4-(1H-1,2,3,4-tetrazol-1-yl), Ascaridole epoxide, 2-Methoxy-4-vinylphenol, 6-epi-shyobunol, Phenol,2-methoxy-5-(1-propenyl)-,E, Alfa.-Copaene, 8-Isopropenyl-1,5-dimethyl-cyclodeca-1,5-diene, Bicyclo[3.1.0]hexane-6-methanol,2-hydroxy-1,4,4-trimethyl, 7-epi-cis-sesquisabinene hydrate, Alloaromadendrene, Benzene,1-(1,5-dimethyl-4-hexenyl)-4-methyl, 1,3-Cyclohexadiene ,5-(1,5-dimethyl-4-hexenyl)-2methyl-,[S-(R*,S*)], Aromadendrene oxide, 1,6,10-Dodecatrien-3-ol,3,7,11-trimethyl-,(E), 4-((1H)-3-Hydroxy-1-propenyl)-2-methoxyphenol, Butan-2-one,4-(3-hydroxy-2-methoxyphenyl), Longipinocarveol,trans, Cholestan-3-ol,2-methylene-,(3ß,5α)-, Bicyclo[4.4.0]dec-2-ene-4-ol,2-methyl-9-(prop-1-en-3-ol-2-yl)-, Corymbolone, Estra-1,3,5(10)-trien-17ß-ol, 1-Heptatriacotanol, Fenretinide, Folic acid, Spiro[4.5]decan-7-one,1,8-dimethyl-8,9-epoxy-4-isopropyl-, 7H-6,9a-Methano-4H-cyclopenta[9,10] cyclopropa[5,6]cyclodeca[1, Gingerol, 1b,4a-Epoxy-2H-cyclopenta[3,4]cyclopropa [8,9]cycloundec[1,2-b]o, Cyclopropa[5,6]-A-nor-5α-androstane-3,7-dione,3´,6ß-dihydro-17ß-h, Olean-12-ene-3,15,16,21,22,28-hexol,(3ß,15α,16α,21ß,22α)-, Benz[e]azulen-3(3aH)-one,4,6a,7,8,9,10,10a,10b-octahydro-3a,8,1, Naphthalene, decahydro-1-pentadecyl-, 13-Docosenamide,(Z)-, 9,10-Secocholesta-5,7,10(19)-triene-3,24,25-triol, (3ß,5Z,7E)-, n-(2,4-Dinitrophenyl)-N´-13-(2,6,6-trimethyl-cyclohex-1-enyl)propylider, n-(2,4-Dinitrophenyl)-N´-13-(2,6,6-trimethyl-cyclohex-1-enyl)propylider, Ingol 12-acetate, 2,2,4-Trimethyl-3-(3,8,12,16-tetramethyl-heptadeca-3,7,11,15-tetrae, Piperine, 2-Methylcortisol, 9-Desoxo-9-x-acetoxy-3,8,12,-tri-O-acetylingol and Propanoic acid ,2-(3-acetoxy-4,4,14-trimethylandrost-8-en-17-yl. (Figure 4-50). In this study five clinical pathogens selected for antibacterial activity namely, (staphylococcus aeureus, klebsiella pneumoniae, pseudomonas aeroginosa, E.coli. and Proteus mirabilis. Maximum zone formation against Klebsiella pneumoniae, Table 2.
 |
Table 1: Bioactive Chemical Compounds Identified In Methanolic Extract Of Zingiber Officinale. |
Table 2: Zone Of Inhibition (Mm) Of Test Bacterial Strains To Zingiber Officinale Bioactive Compounds And Standard Antibiotics.
|
Bacteria |
Plant (Zingiber officinale) / Antibiotics |
|||
|
Zingiber officinale |
Streptomycin |
Rifambin |
Cefotoxime |
|
| Pseudomonaseurogenosa | 4.01±0.188 |
0.92±0.210 |
1.04±0.300 |
1.183±0.100 |
| Escherichiacoli | 2.99±0.311 |
1.500±0.141 |
0.99±0.240 |
2.20±0.170 |
| Klebsiellapneumonia | 4.93±0.290 |
2.02±0.361 |
1.05±0.161 |
0.93±0.150 |
|
Staphylococcus aureus |
3.75±0.910 |
1.00±0.102 |
2.00±0.140 |
1.00±0.301 |
| Proteus
mirabilis |
1.99±0.200 |
2.00±0.180 |
2.06±0.300 |
0.89±0.210 |
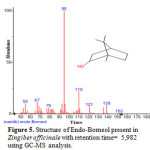 |
Figure 3: Structure of 2-Naphthalenamine,1,2,4a,5,6,7,8,8a-octahydro-4a-methyl present in Zingiber officinale with retention time= 4.987 using GC-MS analysis. |
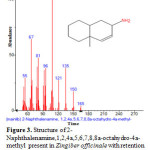 |
Figure 4: Structure of 1-(Cyclopropyl-nitro-methyl)-cyclopentanol present in Zingiber officinale with retention time= 5.702 using GC-MS analysis. |
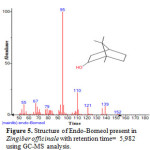 |
Figure 5: Structure of Endo-Borneol present in Zingiber officinale with retention time= 5,982 using GC-MS analysis. |
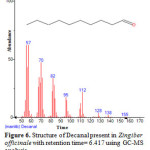 |
Figure 6: Structure of Decanal present in Zingiber officinale with retention time= 6.417 using GC-MS analysis.
|
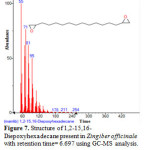 |
Figure 7: Structure of 1,2-15,16-Diepoxyhexadecane present in Zingiber officinale with retention time= 6.697 using GC-MS analysis. |
 |
Figure 8: Structure of Propanal,2-methyl-3-phenyl present in Zingiber officinale with retention time= 6.932 using GC-MS analysis.
|
 |
Figure 9: Structure of Benzeneacetic acid ,4-(1H-1,2,3,4-tetrazol-1-yl) present in Zingiber officinale with retention time= 7.338 using GC-MS analysis.
|
 |
Figure 10: Structure of Ascaridole epoxide present in Zingiber officinale with retention time= 7.750 using GC-MS analysis. |
 |
Figure 11: Structure of 2-Methoxy-4-vinylphenol present in Zingiber officinale with retention time= 7.928 using GC-MS analysis. |
 |
Figure 12: Structure of 6-epi-shyobunol present in Zingiber officinale with retention time= 8.208 using GC-MS analysis. Click here to View figure |
 |
Figure 13: Structure of Phenol,2-methoxy-5-(1-propenyl)-,E present in Zingiber officinale with retention time= 8.414 using GC-MS analysis Click here to View figure |
 |
Figure 14: Structure of 6-epi-shyobunol present in Zingiber officinale with retention time= 8.614 using GC-MS analysis. Click here to View figure |
 |
Figure 15: Structure of Alfa.-Copaene present in Zingiber officinale with retention time= 8.717 using GC-MS analysis. Click here to View figure |
 |
Figure 16: Structure of 8-Isopropenyl-1,5-dimethyl-cyclodeca-1,5-diene present in Zingiber officinale with retention time= 8.900 using GC-MS analysis. Click here to View Figure |
![Figure 17. Structure of Bicyclo[3.1.0]hexane-6-methanol,2-hydroxy-1,4,4-trimethyl present in Zingiber officinale with retention time= 9.055 using GC-MS analysis.](http://www.orientjchem.org/wp-content/uploads/2016/05/Vol32_No2_Anti_Has_Fig17-150x150.jpg) |
Figure 17: Structure of Bicyclo[3.1.0]hexane-6-methanol,2-hydroxy-1,4,4-trimethyl present in Zingiber officinale with retention time= 9.055 using GC-MS analysis. Click here to View figure |
 |
Figure 18: Structure of 7-epi-cis-sesquisabinene hydrate present in Zingiber officinale with retention time= 9.335 using GC-MS analysis. Click here to View figure |
 |
Figure 19: Structure of Alloaromadendrene present in Zingiber officinale with retention time= 9.845 using GC-MS analysis. Click here to View figure |
 |
Figure 20: Structure of Benzene,1-(1,5-dimethyl-4-hexenyl)-4-methyl present in Zingiber officinale with retention time= 10.037 using GC-MS analysis. Click here to View figure |
![Figure 21. Structure of 1,3-Cyclohexadiene ,5-(1,5-dimethyl-4-hexenyl)-2methyl-,[S-(R*,S*)] present in Zingiber officinale with retention time= 10.228 using GC-MS analysis.](http://www.orientjchem.org/wp-content/uploads/2016/05/Vol32_No2_Anti_Has_Fig21-150x150.jpg) |
Figure 21: Structure of 1,3-Cyclohexadiene ,5-(1,5-dimethyl-4-hexenyl)-2methyl-,[S-(R*,S*)] present in Zingiber officinale with retention time= 10.228 using GC-MS analysis. Click here to View figure |
 |
Figure 22: Structure of Aromadendrene oxide present in Zingiber officinale with retention time= 10.869 using GC-MS analysis. Click here to View figure |
 |
Figure 23: Structure of 1,6,10-Dodecatrien-3-ol,3,7,11-trimethyl-,(E) present in Zingiber officinale with retention time= 10.972 using GC-MS analysis. Click here to View figure |
 |
Figure 24: Structure of 4-((1H)-3-Hydroxy-1-propenyl)-2-methoxyphenol present in Zingiber officinale with retention time= 11.224 using GC-MS analysis. Click here to View figure |
 |
Figure 25: Structure of Butan-2-one,4-(3-hydroxy-2-methoxyphenyl) present in Zingiber officinale with retention time= 12.248 using GC-MS analysis. Click here to View figure |
 |
Figure 26: Structure of Longipinocarveol,trans present in Zingiber officinale with retention time= 12.631 using GC-MS analysis. Click here to View figure |
 |
Figure 27: Structure of Cholestan-3-ol,2-methylene-,(3ß,5α) present in Zingiber officinale with retention time= 12.837 using GC-MS analysis. Click here to View figure |
![Figure 28. Structure of Bicyclo[4.4.0]dec-2-ene-4-ol,2-methyl-9-(prop-1-en-3-ol-2-yl) present in Zingiber officinale with retention time= 13.427 using GC-MS analysis.](http://www.orientjchem.org/wp-content/uploads/2016/05/Vol32_No2_Anti_Has_Fig28-150x150.jpg) |
Figure 28: Structure of Bicyclo[4.4.0]dec-2-ene-4-ol,2-methyl-9-(prop-1-en-3-ol-2-yl) present in Zingiber officinale with retention time= 13.427 using GC-MS analysis. Click here to View figure |
 |
Figure 29: Structure of Corymbolone present in Zingiber officinale with retention time= 14.268 using GC-MS analysis. Click here to View figure |
 |
Figure 30: Structure of Estra-1,3,5(10)-trien-17ß-ol present in Zingiber officinale with retention time= 15.240 using GC-MS analysis. Click here to View figure |
 |
Figure 31: Structure of 1-Heptatriacotanol present in Zingiber officinale with retention time= 15.166 using GC-MS analysis. Click here to View figure |
 |
Figure 32: Structure of Fenretinide present in Zingiber officinale with retention time= 16.059 using GC-MS analysis. Click here to View figure |
 |
Figure 33: Structure of Folic acid present in Zingiber officinale with retention time= 15.675 using GC-MS analysis Click here to View figure |
![Figure 34. Structure of Spiro[4.5]decan-7-one,1,8-dimethyl-8,9-epoxy-4-isopropyl present in Zingiber officinale with retention time= 15.034 using GC-MS analysis.](http://www.orientjchem.org/wp-content/uploads/2016/05/Vol32_No2_Anti_Has_Fig34-150x150.jpg) |
Figure 34: Structure of Spiro[4.5]decan-7-one,1,8-dimethyl-8,9-epoxy-4-isopropyl present in Zingiber officinale with retention time= 15.034 using GC-MS analysis. Click here to View figure |
![Figure 35. Structure of 7H-6,9a-Methano-4H-cyclopenta[9,10]cyclopropa[5,6]cyclodeca[1, present in Zingiber officinale with retention time= 17.495 using GC-MS analysis.](http://www.orientjchem.org/wp-content/uploads/2016/05/Vol32_No2_Anti_Has_Fig35-150x150.jpg) |
Figure 35: Structure of 7H-6,9a-Methano-4H-cyclopenta[9,10]cyclopropa[5,6]cyclodeca[1, present in Zingiber officinale with retention time= 17.495 using GC-MS analysis. Click here to View figure |
 |
Figure 36: Structure of Gingerol present in Zingiber officinale with retention time= 18.799 using GC-MS analysis. Click here to View figure |
![Figure 37. Structure of 1b,4a-Epoxy-2H-cyclopenta[3,4]cyclopropa[8,9]cycloundec[1,2-b]o present in Zingiber officinale with retention time= 18.897 using GC-MS analysis.](http://www.orientjchem.org/wp-content/uploads/2016/05/Vol32_No2_Anti_Has_Fig37-150x150.jpg) |
Figure 37: Structure of 1b,4a-Epoxy-2H-cyclopenta[3,4]cyclopropa[8,9]cycloundec[1,2-b]o present in Zingiber officinale with retention time= 18.897 using GC-MS analysis. Click here to View figure |
![Figure 38. Structure of Cyclopropa[5,6]-A-nor-5α-androstane-3,7-dione,3´,6ß-dihydro-17ß-h present in Zingiber officinale with retention time= 19.795 using GC-MS analysis.](http://www.orientjchem.org/wp-content/uploads/2016/05/Vol32_No2_Anti_Has_Fig38-150x150.jpg) |
Figure 38: Structure of Cyclopropa[5,6]-A-nor-5α-androstane-3,7-dione,3´,6ß-dihydro-17ß-h present in Zingiber officinale with retention time= 19.795 using GC-MS analysis. Click here to View figure |
 |
Figure 39: Structure of Olean-12-ene-3,15,16,21,22,28-hexol,(3ß,15α,16α,21ß,22α) present in Zingiber officinale with retention time= 20.533 using GC-MS analysis. Click here to View figure |
![Figure 40. Structure of Benz[e]azulen-3(3aH)-one,4,6a,7,8,9,10,10a,10b-octahydro-3a,8,1 present in Zingiber officinale with retention time= 20.768 using GC-MS analysis.](http://www.orientjchem.org/wp-content/uploads/2016/05/Vol32_No2_Anti_Has_Fig40-150x150.jpg) |
Figure 40: Structure of Benz[e]azulen-3(3aH)-one,4,6a,7,8,9,10,10a,10b-octahydro-3a,8,1 present in Zingiber officinale with retention time= 20.768 using GC-MS analysis. Click here to View figure |
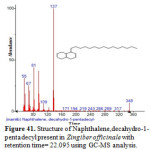 |
Figure 41: Structure of Naphthalene,decahydro-1-pentadecyl present in Zingiber officinale with retention time= 22.095 using GC-MS analysis. Click here to View figure |
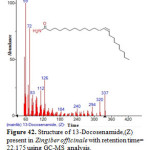 |
Figure 42: Structure of 13-Docosenamide,(Z) present in Zingiber officinale with retention time= 22.175 using GC-MS analysis. Click here to View figure |
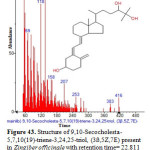 |
Figure 43: Structure of 9,10-Secocholesta-5,7,10(19)-triene-3,24,25-triol, (3ß,5Z,7E) present in Zingiber officinale with retention time= 22.811 using GC-MS analysis. Click here to View figure |
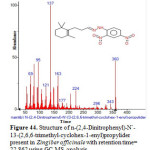 |
Figure 44: Structure of n-(2,4-Dinitrophenyl)- ´-13-(2,6,6-trimethyl-cyclohex-1-enyl)propylider present in Zingiber officinale with retention time= 22.862 using GC-MS analysis. Click here to View figure |
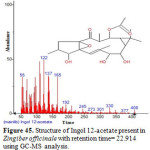 |
Figure 45: Structure of Ingol 12-acetate present in Zingiber officinale with retention time= 22.914 using GC-MS analysis. Click here to View figure |
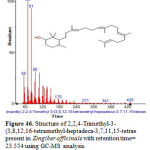 |
Figure 46: Structure of 2,2,4-Trimethyl-3-(3,8,12,16-tetramethyl-heptadeca-3,7,11,15-tetrae present in Zingiber officinale with retention time= 23.554 using GC-MS analysis. Click here to View figure |
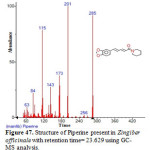 |
Figure 47: Structure of Piperine present in Zingiber officinale with retention time= 23.629 using GC-MS analysis. Click here to View figure |
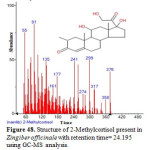 |
Figure 48: Structure of 2-Methylcortisol present in Zingiber officinale with retention time= 24.195 using GC-MS analysis. Click here to View figure |
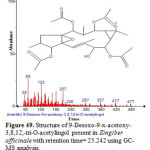 |
Figure 49: Structure of 9-Desoxo-9-x-acetoxy-3,8,12,-tri-O-acetylingol present in Zingiber officinale with retention time= 25.242 using GC-MS analysis. Click here to View figure |
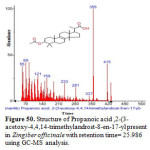 |
Figure 50: Structure of Propanoic acid ,2-(3-acetoxy-4,4,14-trimethylandrost-8-en-17-yl present in Zingiber officinale with retention time= 25.986 using GC-MS analysis. Click here to View figure |
Conclusion
From the results obtained in this study, it could be concluded that Zingiber officinale possesses remarkable antimicrobial activity, which is mainly due to naphthalenamine, decanal, and alfa.-copaene. According to these findings, it could be said that the methanolic extract act as antibacterial agents.
Acknowledgements
Special thanks to Prof. Abdul-Kareem, Babylon University, Faculty of science for women, for his special care.
References
- Ross, I.A. Medicinal Plants of the World. Humana Press, Totowa, New Jersey: 507-560.
- Chioma, A.A.; Onyechi, O.; Lawrence, U.S.; Meshach, M.N. African Journal of Biochemistry Research. 2009, 3(12), 379-384.
- Ahmed, R.S.; Seth, V.; Banergee, B.D. Indian J Exp Biol. 2000, 38(6), 604-6.
- Ojewole, J.A. Phyother. Res. 2006, 20, 764-772.
CrossRef - Nurtijahja, T.E.; Ammit, A.J., Roufoglis, B.D., Tran, V.H., Duke, C.C. Thromb. Res. 2003, 111, 259-265.
CrossRef - Chaieb, N., Gonzales, J.L.; Lopez-Mesas, M.; Bouslama, M.; Valiente, M. Food Research International. 2011, 44(4), 970-977.
CrossRef - Xu, B.J.; Yuan, S.H.; Chang, S.K.C. Journal of Food Science. 2007, 72(2), 167–177.
CrossRef - Ficker, C.E.; Arnason, J.T.; Vindas, P.S.; Alvarez, L.P.; Akpagana, K.; Gbeassor, M.; DeSouza, C.; Smith, M.L. Mycoses. 2003, 46: 29-37.
CrossRef - Al-Marzoqi, A.H.; Hameed, I.H.; Idan, S.A. African Journal of Biotechnology.2015, 14(40), 2812-2830.
CrossRef - Khushtar, M.; Kumar, V.; Javed, K., Uma, B. (2009). Indian J Pharm Sci. 1(5): 554-558.
- Altameme, H.J.; Hadi, M.Y.; Hameed, I.H. Journal of Pharmacognosy and Phytotherapy. 2015a, 7(10), 238-252.
CrossRef - Habib, S.H.; Makpol, S.; Hamid, N.A.; Das, S.; Ngah, W.Z.; Yusof, Y.A. Clinics. 2008, 63: 807-813.
CrossRef - Altameme, H.J.; Hameed, I.H.; Idan, S.A.; Hadi, M.Y. Journal of Pharmacognosy and Phytotherapy. 2015b, 7(9), 221-237.
CrossRef - Hamza, L.F.; Kamal, S.A.; Hameed, I.H. Journal of Pharmacognosy and Phytotherapy. 2015, 7(9), 194-220.
CrossRef - Altameme, H.J.; Hameed, I.H.; Kareem, M.A. African Journal of Biotechnology. 2015c, 14(19), 1668-1674.
- Jasim, H.; Hussein, A.O.; Hameed, I.H.; Kareem, M.A. Journal of Pharmacognosy and Phytotherapy. 2015, 7(4): 56-72.
- Mohammed, A.; Imad, H. Research Journal of Biotechnology. 2013, 8(10), 92-105.
- Hameed, I.H.; Abdulzahra, A.I.; Jebor, M.A.; Kqueen, C.Y.; Ommer, A.J. Mitochondrial DNA. 2015a , 26(4), 544-9.
CrossRef - Hameed, I.H.; Hamza L.F.; Kamal S.A. Journal of Pharmacognosy and Phytotherapy. 2015b, 7(8), 132-163.
CrossRef - Muhanned, A.K.; Ameer, I.A.; Imad, H.H.; Mohammed, A.J. Mitochondrial DNA. 2015, 1–5.
- Hameed, I.H.; Hussein, H.J.; Kareem, M.A.; Hamad, N.S. Journal of Pharmacognosy and Phytotherapy. 2015c, 7(7), 107-125.
CrossRef - Hameed, I.H.; Jebor, M.A.; Ommer, A.J.; Abdulzahra, A.I.; Yoke, C. Mitochondrial DNA. 2014, 4:1-4.
- Idan, S.A.; Al-Marzoqi, A.H.; Hameed, I.H. Journal of Pharmacognosy and Phytotherapy. 2015, 14(46), 3131-3158.
- Hameed, I.H.; Ibraheam, I.A.; Kadhim, H.J. Journal of Pharmacognosy and Phytotherapy. 2015d, 7(6), 90-106.
CrossRef - http://www.orientjchem.org/wp-content/uploads/2016/05/Vol32_No2_Anti_Has_Fig43.jpg

This work is licensed under a Creative Commons Attribution 4.0 International License.









