Antibacterial Activity and Global Reactivity Descriptors of Some Newly Synthesized Unsymmetrical Sulfamides
Amel Bendjeddou1,*, Tahar Abbaz1,3, Adel Ayari1, Merzoug Benahmed2, Abdelkrim Gouasmia3 and Didier Villemin4
1Laboratory of Aquatic and Terrestrial Ecosystems, Organic and Bioorganic Chemistry group, University of Souk Ahras, Algeria 2Laboratory of Bioactive Molecules and Applications, University of Tebessa, Algeria 3Laboratory of Organic Materials and Heterochemistry, University of Tebessa, Algeria 4Laboratory of Molecular and Thio-Organic Chemistry, UMR CNRS 6507, INC3M, FR 3038, Labex EMC3, ensicaen and University of Caen, Caen 14050, France Corresponding Author E-mail: bendjeddouamel@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/320205
Article Received on :
Article Accepted on :
Article Published : 04 Apr 2016
In the present study a series of unsymmetric linear sulfamides (1-9) starting from a primary amine were synthesized and their structures were confirmed by elemental analyses, mass spectrometry and 1H NMR techniques. All the synthesized compounds were screened for their antibacterial activities by both disc diffusion and minimal inhibition concentration (MIC) methods. Frontier molecular orbital (FMO) analysis and global reactivity descriptors have been performed using the density functional theory (DFT) with the B3LYP functional. The results indicated that these derivatives, depending of their substituted radical, bring about an improvement in the bacterial activity.
KEYWORDS:Unsymmetric sulfamides; Antibacterial activity; global reactivity; Minimum inhibitory concentrations (MIC)
Download this article as:| Copy the following to cite this article: Bendjeddou A, Abbaz T, Ayari A, Benahmed M, Gouasmia A, Villemin D. Antibacterial Activity and Global Reactivity Descriptors of Some Newly Synthesized Unsymmetrical Sulfamides. Orient J Chem 2016;32(2). |
| Copy the following to cite this URL: Bendjeddou A, Abbaz T, Ayari A, Benahmed M, Gouasmia A, Villemin D. Antibacterial Activity and Global Reactivity Descriptors of Some Newly Synthesized Unsymmetrical Sulfamides. Orient J Chem 2016;32(2). Available from: http://www.orientjchem.org/?p=15169 |
Introduction
Sulfonamides (sulfa drugs) were the first drugs largely employed and systematically used as preventive and chemotherapeutic agents against various diseases1. They are active against a broad range of Gram-positive and Gram-negative bacteria and function as competitive antagonists to bacterial folate synthesis2. They constitute an important class of drugs, with several types of pharmacological activities including antibacterial3 anti-carbonic anhydrase4 diuretic5 hypoglycemic6 and antithyroid activity7. Sulfonamides were primarily developed as antibacterial agents, with sulfanilamide the first recognized sulfonamide antibacterial. Since then many other effective antibacterial derived from sulfonamides have been discovered and utilized in medicine. These classes of compounds are considered as “scalffolds” in medicinal chemistry to drug development with different biological activities. In organic chemistry, these compounds have a functional application in the industry in some products of health, food colorants and others; therefore it is necessary to continue with research projects that help to synthesize new compounds with sulfonamide group.
In light of these, we became interested in the synthesis, characterization and biological evaluation of unsymmetric linear sulfamides. In order to assess more accurately and to provide a background frame work for the work described in this paper, a correlation between biological activity and some appropriate quantum descriptors8-9 such as EHOMO, ELUMO, energy gap, global hardness, global hardness softness, electrophilicity index and molecular electrostatic potential have also been carried out from the density functional theory (DFT).
Experimental Section
General
NMR spectra were recorded on a WP 400-NMR instrument. FAB mass spectra were recorded on a JOEL JMS-DX 300 spectrometer. Uncorrected melting points were measured on a 510 Buchi apparatus. Density functional theory calculations were carried out using the Gaussian 09W program packages developed by Frisch and coworkers10. The Becke’s three parameter hybrid functional using the LYP correlation functional (B3LYP), one of the most robust functional of the hybrid family, was herein used for all the calculations, with 6.31G (d, p) basis set11-12. Gaussian output files were visualized by means of gaussian view 05 software13. All solvents were dried by standard methods and all commercial reagents used without purification. All reactions were performed under an inert atmosphere of nitrogen.
Inhibition zones (DZI) of the compounds were examined by disc diffusion technique14-15. The Antibacterial screening was performed using Mueller–Hinton agar for 24 hours at 37°C. After incubation, the zone of growth inhibition around the disks was measured in millimeter (mm). All tests were performed in duplicate, and experiment was repeated three times.
Minimum Inhibitory Concentrations (MICs) values, defined as the lowest concentration of sample which inhibits the visible growth of microorganism after overnight incubation, were also determined by the broth dilution method following the procedures recommended by the CLSI (Clinical and Laboratory Standarts Institute)14.
Synthesis and Characterization of unsymmetrical sulfamides 1-9
A solution of sulfuryl chloride (1 equiv, 10 mmol, 1.35 g) in hexane (30 ml) was added dropwise to a stirred solution of the first amine (1 equiv, 10 mmol) and the second amine (1 equiv, 10 mmol) in hexane (80 ml) cooled to 0°C. The reaction mixture was stirred for 6h, then it was extracted with CH2Cl2 (200 ml), the organic phase was washed with 120 ml of 1M HCl, followed with water (120 ml), and dried with Na2SO4 and the solvent was removed under reduced pressure to give the crude as colorless oil. Compounds 1-9 were obtained by column chromatography of the residue (silica gel, eluting with (AcOEt / n-Hexane: 6/4).
N-(thiophen-2-yl methyl)-N’-(tert-butyl)sulfamide 1
Beige powder. Yield: 25%. M.p.: 155°C. TLC: Rf = 0.74 (AcOEt / n-Hexane: 6/4). 1H NMR (400 MHz, CDCl3, δ, ppm): 1.40 (s, 9H, tBu); 3.93 (s, 2H, CH2-N-thio); 4.30 (s, 1H, NH-tBu); 4.45 (s, 1H, NH-thio); 6.63 (d, 1H, J = 3.60 Hz, thio ); 6.75 (t,1H, J = 4.45 Hz, thio); 6.95 (d, 1H, J = 4.45 Hz, thio). MS (NOBA, FAB > 0): 627 [M + H]+. Anal. calcd for C9H16N2O2S2: C, 43.52%; H, 6.49; S, 25.82%; found: C, 43.67%; H, 6.59%; S, 25.60%.
N-(thiophen-2-yl methyl)-N’-(isopentyl)sulfamide 2
Beige powder. Yield: 28%. M.p.: 149°C. TLC: Rf = 0.80 (AcOEt / n-Hexane: 6/4). 1H NMR (400 MHz, CDCl3, δ, ppm): 1.10 (d, 6H, 2CH3); 1.60 (m, 2H, CH2); 1.90 (m, 1H, CH-(CH3)2); 2.75 (t, 2H, CH2-N); 4.26 (s, 1H, NH-isopro); 4.45 (s, 1H, NH-thio); 3.93 (s, 2H, CH2-N-thio); 6.63 (d, 1H, J = 3.60 Hz, thio); 6.75 (t,1H, J = 4.45 Hz, thio); 6.95 (d, 1H, J = 4.45 Hz, thio ). MS (NOBA, FAB > 0): 627 [M + H]+. Anal. calcd for C10H18N2O2S2: C, 45.77%; H, 6.91; S, 24.44%; found: C, 46.00%; H, 7.10%; S, 24.25%.
N-(thiophen-2-yl methyl)-N’-(isobutyl)sulfamide 3
Beige powder. Yield: 26%. M.p.: 151°C. TLC: Rf = 0.77 (AcOEt / n-Hexane: 6/4). 1H NMR (400 MHz, CDCl3, δ, ppm): 1.06 (d, 6H, 2CH3); 2.00 (m, 1H, CH(CH3)2); 2.65 (d, 2H, CH2-N); 3.93 (s, 2H, CH2-N-thio); 4.28 (s, 1H, NH-iBu); 4.45 (s, 1H, NH-thio); 6.63 (d, 1H, J = 3.60 Hz, thio ); 6.75 (t,1H, J = 4.45 Hz, thio); 6.95 (d, 1H, J = 4.45 Hz, thio). MS (NOBA, FAB > 0): 627 [M + H]+. Anal. calcd for C9H16N2O2S2: C, 43.52%; H, 6.49; S, 25.82%; found: C, 43.77%; H, 6.58%; S, 25.53%.
N-(pyridin-4-yl methyl)-N’-(tert-butyl)sulfamide 4
Scarcely green powder. Yield: 30%. M.p.: 147°C. TLC: Rf = 0.61 (AcOEt / n-Hexane: 6/4). 1H NMR (400 MHz, CDCl3, δ, ppm): 1.40 (s, 9H, tBu); 3.96 (d, 2H, J = 4.66 Hz, CH2 -pyr); 4.30 (s, 1H, NH-tBu); 4.50 (s, 1H, NH-pyr) 7.45 (d, 2H, J = 4.65 Hz, pyr); 8.72 (d, 2H, J = 4.68 Hz, pyr). MS (NOBA, FAB > 0): 627 [M + H]+. Anal. calcd for C10H17N3O2S: C, 49.36%; H, 7.04; S, 13.17%; found: C, 49.60%; H, 7.17%; S, 13.25%.
N-(pyridin-4-yl methyl)-N’-(isopentyl)sulfamide 5
Scarcely green powder. Yield: 33%. M.p.: 142°C. TLC: Rf = 0.66 (AcOEt / n-Hexane: 6/4). 1H NMR (400 MHz, CDCl3, δ, ppm): 1.10 (d, 6H, 2CH3); 1.60 (m, 2H, CH2); 1.90 (m, 1H, CH-(CH3)2); 2.75 (t, 2H, CH2-N); 3.96 (d, 2H, J = 4.66 Hz, CH2 -pyr); 4.26 (s, 1H, NH-isopro); 4.50 (s, 1H, NH-pyr) 7.45 (d, 2H, J = 4.65 Hz, pyr); 8.72 (d, 2H, J = 4.68 Hz, pyr). MS (NOBA, FAB > 0): 627 [M + H]+. Anal. calcd for C11H19N3O2S: C, 51.33%; H, 7.44; S, 12.46%; found: C, 51.48%; H, 7.59%; S, 12.61%.
N-(pyridin-4-yl methyl)-N’-(isobutyl)sulfamide 6
Scarcely green powder. Yield: 31%. M.p.: 145°C. TLC: Rf = 0.63 (AcOEt / n-Hexane: 6/4). 1H NMR (400 MHz, CDCl3, δ, ppm): 1.06 (d, 6H, 2CH3); 2.00 (m, 1H, CH(CH3)2); 2.65 (d, 2H, CH2-N); 3.96 (d, 2H, J = 4.66 Hz, CH2-pyr); 4.28 (s, 1H, NH-iBu); 4.50 (s, 1H, NH-pyr) 7.45 (d, 2H, J = 4.65 Hz, pyr); 8.72 (d, 2H, J = 4.68 Hz, pyr). MS (NOBA, FAB > 0): 627 [M + H]+. Anal. calcd for C10H17N3O2S: C, 49.36%; H, 7.04; S, 13.17%; found: C, 49.51%; H, 7..15%; S, 13.22%.
N-(benzyl)-N’-(tert-butyl)sulfamide 7
White powder. Yield: 21%. M.p.: 136°C. TLC: Rf = 0.70 (AcOEt / n-Hexane: 6/4). 1H NMR (400 MHz, CDCl3, δ, ppm): 1.40 (s, 9H, tBu); 4.15 (d, 2H, J = 5.99 Hz, CH2 -Ph); 4.30 (s, 1H, NH-tBu); 4.47 (s, 1H, NH-Bn); 7.30 (m, 5H, ArH). MS (NOBA, FAB > 0): 627 [M + H]+. Anal. calcd for C11H18N2O2S: C, 54.51%; H, 7.48; S, 13.23%; found: C, 54.67%; H, 7.59%; S, 13.35%.
N-(benzyl)-N’-(isopentyl)sulfamide 8
White powder. Yield: 24%. M.p.: 130°C. TLC: Rf = 0.75 (AcOEt / n-Hexane: 6/4). 1H NMR (400 MHz, CDCl3, δ, ppm): 1.10 (d, 6H, 2CH3); 1.60 (m, 2H, CH2); 1.90 (m, 1H, CH-(CH3)2); 2.75 (t, 2H, CH2-N); 4.15 (d, 2H, J = 5.99 Hz, CH2 -Ph); 4.26 (s, 1H, NH-isopro); 4.47 (s, 1H, NH-Bn); 7.30 (m, 5H, ArH) . MS (NOBA, FAB > 0): 627 [M + H]+. Anal. calcd for C12H20N2O2S: C, 56.22%; H, 7.86; S, 12.50%; found: C, 56.33%; H, 8.02%; S, 12.43%.
N-(benzyl)-N’-(isobutyl)sulfamide 9
White powder. Yield: 22%. M.p.: 133°C. TLC: Rf = 0.73 (AcOEt / n-Hexane: 6/4). 1H NMR (400 MHz, CDCl3, δ, ppm): 1.06 (d, 6H, 2CH3); 2.00 (m, 1H, CH(CH3)2); 2.65 (d, 2H, CH2-N); 4.15 (d, 2H, J = 5.99 Hz, CH2 -Ph); 4.28 (s, 1H, NH-iBu); 4.47 (s, 1H, NH-Bn); 7.30 (m, 5H, ArH). MS (NOBA, FAB > 0): 627 [M + H]+. Anal. calcd for C11H18N2O2S: C, 54.51%; H, 7.48; S, 13.23%; found: C, 54.43%; H, 7.63%; S, 13.31%.
Results and Discussion
Synthesis
In this work, we exploit a method developed in previous works16-17, for the preparation of unsymmetrical sulfamides derived of primary amines. Unsymmetrical and symmetrical sulfamides can be prepared by the direct addition of sulfuryl chloride to a mixture of two primary amines in cyclohexane or hexane at 0°C. As discussed in our previous work, we have also obtained three compounds: two symmetric sulfamides and the mixed sulfamide. TLC reveals unsymmetrical sulfamide formation and it is located in the middle between the first and the second symmetric sulfamides. In this paper, we are interested to the separation of dissymmetric sulfamides in order to estimate their antibacterial activity.
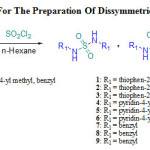 |
Scheme 1: Synthetic Route For The Preparation Of Dissymmetric Sulfamides Derivatives 1-9 |
In the 1H NMR spectra all synthetized compounds revealed the presence of amino group protons signals as singlet around 4.26 – 4.45 ppm, 4.26 – 4.47 ppm and 4.26 – 4.50 ppm for the thiophene, benzyl and pyridine series, respectively. Mass spectrometry analysis validated the structure of the examined derivatives. In all compounds, fragmentation peaks confirmed the structure of the analyzed molecules.
 |
Figure 1: Antibacterial activity against Gram– and Gram+ strains |
Table 1: Zones Of Growth Inhibition And MIC Values Of The Compounds 1-9
|
Bacterial strains |
Enterobacteriaceae |
Staphylococcus aures |
|
|
1 |
DZI (mm) |
20 |
24 |
|
MIC (µg/ml) |
64 |
16 |
|
|
2 |
DZI (mm) |
18 |
22 |
|
MIC (µg/ml) |
4 |
2 |
|
|
3 |
DZI (mm) |
18 |
20 |
|
MIC (µg/ml) |
32 |
4 |
|
|
4 |
DZI (mm) |
33 |
36 |
|
MIC (µg/ml) |
512 |
256 |
|
|
5 |
DZI (mm) |
31 |
31 |
|
MIC (µg/ml) |
64 |
64 |
|
|
6 |
DZI (mm) |
30 |
30 |
|
MIC (µg/ml) |
256 |
128 |
|
|
7 |
DZI (mm) |
14 |
18 |
|
MIC (µg/ml) |
512 |
256 |
|
|
8 |
DZI (mm) |
13 |
16 |
|
MIC (µg/ml) |
64 |
32 |
|
|
9 |
DZI (mm) |
13 |
13 |
|
MIC (µg/ml) |
512 |
128 |
|
It was evident From Table 1 That the compound 2 of Series 1 (thiophene moiety) containing isopentyl substituted have exhibited higher activity than the compound 3 containing substituted isobutyl moiety followed by the compound 1 containing substituted tert-butyl moiety, their MIC values were (4 – 32 – 64 μg/mL) against Enterobacteriaceae and (2 – 4 – 16 μg/mL) against Staphylococcus aures, respectively.
Series 2 (pyridine moiety), among compounds 4, 5, and 6 derived from pyridine, compound 5 was the most potent among their series with MIC equal to 64 μg/ml (31mm) , while the compounds 4 and 6 showed moderate antibacterial activity with a MIC value equal to 128 – 512 µg/ml against Staphylococcus Aures and Enterobacteriaceae.
Series 3 (benzyl moiety), compounds 7 and 9 with tert-butyl and isobutyl as alkyls, respectively showed moderate activity, their MIC values were (128 – 512 µg/ml), Whereas compound 8 which also contain a benzyl moiety but with isopentyl alkyl was the most active among this series with a MIC value equal to 32 – 64 µg/ml towards Staphylococcus aures and Enterobacteriaceae, respectively.
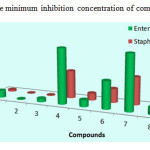 |
Figure 2: The minimum inhibition concentration of compounds 1-9 |
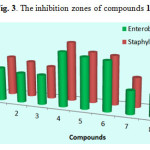 |
Figure 3: The inhibition zones of compounds 1-9 |
In conclusion, Antibacterial Activity studies indicate that isopentyl substituted sulfonamides of each series were more active than the other members.
Theoretical calculation
The highest occupied molecular orbital (HOMO), the lowest unoccupied molecular orbital (LUMO) and HOMO and LUMO energy gaps for compounds 1-9 calculated at DFT level in the 6-31G basis set. The eigenvalues of LUMO and HOMO and their energy gap reflect the chemical activity of the molecule. LUMO as an electron acceptor represents the ability to obtain an electron, while HOMO as an electron donor represents the ability to donate an electron. The smaller the LUMO and HOMO energy gaps, the easier it is for the HOMO electrons to be excited; the higher the HOMO energies, the easier it is for HOMO to donate electrons; the lower the LUMO energies, the easier it is for LUMO to accept electrons. A hard molecule has a large energy gap, and a soft molecule has a small gap.
Table 2: Some Energetic Properties Of Compounds 1-9
|
Compounds |
EHOMO (eV) |
ELUMO (eV) |
ΔEgap (eV) |
I (eV) |
A (eV) |
|
1 |
-6.168 |
-0.389 |
5.778 |
6.168 |
0.389 |
|
2 |
-6.218 |
-0.407 |
5.811 |
6.218 |
0.407 |
|
3 |
-6.229 |
-0.411 |
5.818 |
6.229 |
0.411 |
|
4 |
-6.375 |
-0.834 |
5.541 |
6.375 |
0.834 |
|
5 |
-6.476 |
-0.857 |
5.619 |
6.476 |
0.857 |
|
6 |
-6.497 |
-0.863 |
5.634 |
6.497 |
0.863 |
|
7 |
-6.198 |
-0.241 |
5.957 |
6.198 |
0.241 |
|
8 |
-6.296 |
-0.261 |
6.035 |
6.296 |
0.261 |
|
9 |
-6.317 |
-0.268 |
6.049 |
6.317 |
0.268 |
From theoretical calculations established, it was found that the molecule 4 has the lowest energetic gap (ΔEgap = 5.54 eV), so it is the softest molecule and it is the best to be easily excited by against, the molecule 3 has the highest energy gap (ΔEgap = 5.81 eV), so it is the hardest molecule. Molecule 1 has the highest HOMO energy (EHOMO = -6.16 eV) that allows him to be the best electron donor molecule; on the other hand the molecule 6 has the lowest LUMO energy (ELUMO = -0.863 eV) that allows it to be the best electron acceptor molecule.
Two important properties of any molecule (M) are its gas-phase ionization potential (I) and its electron affinity (A). The determination of I and A allows the absolute electronegativity (χ) and absolute hardness (η) parameters for M to be calculated. In the most common case, I and A are related to the one-electron orbital energies of the HOMO and LUMO, respectively. Then (I−A) is simply the difference in energy between the HOMO and the LUMO. Soft molecules have a small energy gap. Low ‘I’ creates a better electron donor and large ‘A’ makes a better electron acceptor. For almost all of the commonly used exchange-correlation functional, the HOMO and LUMO energy are not close to the exact IP and EA respectively but, excellent linear correlation relationship exists between HOMO energies and calculated IP and also between the negative of the LUMO energies and calculated EA. Therefore based on these linear correlation relationships, the calculated HOMO and LUMO energies can be used to semi quantitatively estimate the ionization potential and electron affinity.
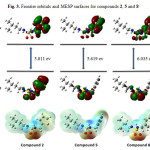 |
Figure 3: Frontier orbitals and MESP surfaces for compounds 2, 5 and 8 |
Considering the above, compound 6 has the greater electron affinity value (A = 0.86 eV) which indicate that it is the best electron acceptor. Compound 1 has the lowest ionization potential value (I = 6.16 eV) which indicate that it is the best electron donor.
Theoretical calculations were performed in order to investigate physico-chemical properties that may be related to the antimicrobial action of the studied compounds. The chemical reactivity of the molecular systems has been determined by the conceptual density functional theory [39]. Electronegativity (ϰ), chemical potential (µ), global hardness (η), global softness (S) and electrophilicity index (ω) are global reactivity descriptors and are highly successful in predicting global reactivity trends. A property of interest in this study was the global electrophilicity index, which may give some insight on the biological activity of compounds.
All these parameters for compounds 1-9 have been listed in Table 3. According to these parameters, the chemical reactivity varies with the structural of molecules. Chemical hardness (softness) value of compound 4 is lesser (greater) among all the molecules. Thus, compound 4 is found to be more reactive than all the molecules. Compound 6 possesses higher electronegativity value than all compounds so; it is the best electron acceptor.
Table 3: The Calculated Quantum Chemical Parameters Of Compounds 1-9
|
Compounds |
µ (eV) |
ϰ (eV) |
η (eV) |
Ѕ (eV) |
ω (eV) |
|
1 |
-3.278 |
3.278 |
2.889 |
0.173 |
1.859 |
|
2 |
-3.312 |
3.312 |
2.905 |
0.172 |
1.887 |
|
3 |
-3.320 |
3.320 |
2.909 |
0.171 |
1.885 |
|
4 |
-3.604 |
3.604 |
2.770 |
0.180 |
2.338 |
|
5 |
-3.666 |
3.666 |
2.809 |
0.178 |
2.392 |
|
6 |
-3.680 |
3.680 |
2.817 |
0.177 |
2.397 |
|
7 |
-3.219 |
3.219 |
2.879 |
0.173 |
1.793 |
|
8 |
-3.278 |
3.278 |
3.017 |
0.166 |
1.784 |
|
9 |
-3.292 |
3.292 |
3.024 |
0.165 |
1.788 |
The values of ω for compounds 1- 9 indicate that they are three series classified in the order, series of pyridine, thiophene and benzyl, successively. The pyridine group has the high value of electrophilicity index which, shows that the compounds of this group are a strong electrophiles than the thiophene and benzyl groups respectively.
Conclusion
In conclusion, a series of novel unsymmetric linear sulfonamides (1-9) were synthesized and their antibacterial activities were evaluated against some bacterial strains. The HOMO, LUMO and MESP surfaces are analyzed to discuss the chemical reactivity patterns in the molecules. A number of reactivity parameters have been calculated to further explain their chemical reactivity. It was observed that within each series, compounds 2, 5 and 8 containing isopentyl alkyl showed the highest biological activity. These new data of these compounds might be helpful in the future development of sulfonamide analogues as novel antibacterial agents.
Acknowledgments
This work was generously supported by the (General Directorate for Scientific Research and Technological Development, DGRS-DT) and Algerian Ministry of Scientific Research.
References
- Hansch C.; Sammes P. G.; Taylor J. B. Comprehensive Medicinal Chemistry, Pergamon Press: Oxford 1990. Vol. 2, Chapter 7.1.
- Vree, T. H. Clinical Pharmacokinetics of Sulfonamides and Their Metabolites. Karger: Basel, 1987. Vol. 37.
- Karale , B. K.; Takate, S. J.; Salve, S. P.; Zaware, B. H. and Jadhav S. S. Orient. J. Chem., 2015, 31, 307-315.
CrossRef - Supuran, C. T.; Scozzafava, A. Curr. Med. Chem. 2001, 1, 61-97.
- Maren, T. H. Annu. Rev. Pharmacol. Toxicol. 1976, 16, 309-327.
CrossRef - Boyd, A. E. Diabetes 1988, 37, 847-850.
CrossRef - Thornber, C. W. Chem. Soc. Rev. 1979, 8, 563-580.
CrossRef - Felegari, Z. Orient. J. Chem., 2014, 30, 1865-1875.
CrossRef - Ouarna, S.; K’tir, H.; Lakrout, S.; Ghorab, H.; Amira, A.; Aouf, Z.; Berredjem, M.; Aouf, N. E. Orient. J. Chem., 2015, 31, 913-919.
CrossRef - Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; et al. Gaussian 09, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2010.
- Schlegel, H. B. J. Comput. Chem. 1982, 3, 214-218.
CrossRef - Ditchfield, R.; Hehre, W. J.; Pople, J. A. J. Chem. Phys. 1971, 54, 724-728.
CrossRef - Dennington, R.; Keith, T.; Millam, J. GaussView, Version 5, Semichem Inc., Shawnee Mission, KS, 2009.
- CLSI document M07-A9. Wayne, PA: Clinical and Laboratory Standards Institute; 2012.
- Venkateswara Rao, J.; Krishna Reddy, V.; Bhavani, R.; Bhavani, B. Orient. J. Chem., 2015, 31, 2253-2258.
CrossRef - Bendjeddou, A.; Djebbar, H.; Berredjem, M.; Hattab, Z.; Regainia, Z.; Aouf, N. E. Phosphorus, Sulfur and Silicon. 2006, 181, 1351-1362.
CrossRef - Bendjeddou, A.; Djeribi, R.; Regainia, Z.; Aouf, N. E. Molecules. 2005, 10, 1387-1398.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









