Synthesis and study of composite organic silica sorption materials
Anna Nikolaevna Shipulya, Elena Vladimirovna Volosova,Elena Valentinovna Pashkova,Natalia Nikolaevna Glazunova and Juliy Alexandrovna Bezgina
The Stavropol State Agrarian University 12, Zootekhnichesky lane, Stavropol, 355017, Russia
corresponding author Email: mtrushin@mail.ru
DOI : http://dx.doi.org/10.13005/ojc/320124
Currently, one of the promising areas of applied chemistry is research and development of composite absorption materials used as sorbents with a wide range of action, as well as media for biologic preparations and drugs. We have performed research on the development of composite organic silica chitosan-silica based materials with certain composition and biochemical action. Silica was used as the main component, and chitosan - as bio-compatible polymer in the composition of the composite sorbent.
KEYWORDS:composite sorbents; chitosan; silica; aerosil
Download this article as:| Copy the following to cite this article: Shipulya A. N, Volosova E. V, Pashkova E. V, Glazunova N. N, Bezgina J. A .Synthesis and study of composite organic silica sorption materials. Orient J Chem 2016;32(1). |
| Copy the following to cite this URL: Shipulya A. N, Volosova E. V, Pashkova E. V, Glazunova N. N, Bezgina J. A .Synthesis and study of composite organic silica sorption materials. Orient J Chem 2016;32(1). Available from: http://www.orientjchem.org/?p=14624 |
Introduction
Multicomponent nanostructures, being composite sorptive materials, currently raise growing interest in various areas of applied chemistry and biotechnology. The sorbents, synthetic polyelectrolytes, and immobilized biosensors obtained with the use of nanotechnology are used as catalysts in solid-phase analytical systems for sorption-spectroscopic rapid determination of objects in the environment, and as highly selective materials for treating biologically active substances in medicine.
Very promising is the obtaining of sorbents with specified composition and structure for their subsequent use in treating biological fluids, and as application sorption materials with controlled adsorption, antimicrobial, and prolonged properties.
Accordingly, it is important to perform research of synthesyzing composite organic and silica materials with the study of their physico-chemical properties, and biochemical action.
One of the methods of obtaining sorption materials is based on obtaining composite sorbents by chemical assembly of structural units and by forming the porous structure of silica matrix in the presence of polymers and highly selective ligands [1].
This area of composite sorbents synthesis allows to create predefined geometric structure of the sorbent, and to activate the surface by organic modifier. In the studies [2,3], high-stable sorbents of standard composition and structure have been obtained, which are specific for a number of biologically active substances (lysozyme, chorionic gonadotropin, hemoglobin). These materials are effective for immobilizing ferments used in biotechnology, medicine, and food industry.
A promising biocompatible polymer in the composition of the sorbents is chitosan [4]. Medical aspects of using chitosan-based preparations are associated with the biological properties of this biopolymer, which has natural origin and known chemical structure. It is biologically compatible and biodegradable to ordinary substances (N – acetylglucosamine or glucosamine), has immunomodulatory, adjuvant, antimicrobial, fungistatic, antitumoral, radioprotective, anti-inflammatory, wound healing, anti-cholesterol, hemostatic effect, and features low toxicity [5].
Chitosan is a fully de-acetylized product of poly [(1-4) – 2 – amino – 2 – deoxy – β – D – glucose]. Usually, complete de-acetylation is not achieved, and chitosan still contains a small amount of N-acetyl-glucosaminidase links. The structure of a chitosan sample with the molecular weight MW=200,000, the deacetylation degree of 0.8 and the degree of crystallinity of 75% is shown in Figure 1:
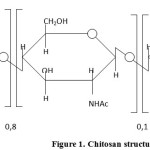 |
Figure 1: Chitosan structure Click here to View figure |
Using the method of IR spectroscopy, it has been established that in the process of chitosan formation, the intensity of carbonyl bands (1,625 cm-1) and amide groups (3,265 and 3,100 cm) absorption decreases, and the intensity of bands at 3,365 and 3,445 cm-1 increases, indicating emergence of an NH2 group [6]. X-ray study of chitosan has shown that it has the same crystal lattice as chitin, but lower regularity of the macromolecules. The chitosan regenerated as film in the salt or the alkali form has the X-ray pattern that is characteristic of amorphous substances.
Unlike chitin, chitosan is soluble in diluted acids, and can be separated from them without change. Therefore, chitosan may be characterized by its viscosity and polydispersity. Work of K. Ogawa [7] has shown that chitosan chloride solution features structural viscosity. Also X-ray diffraction analysis was used for studying some inorganic and organic acid based salts of chitosan. It has been established that a chitosan molecule takes two- or eight-plicated helical conformations, depending on the acid. The salts where the chitosan chain is present in the form of a two-plicated spiral are formed by acids HBr, HI, HNO3, D – and L-ascorbic acids. Eight-plicated spirals are formed by HF, HCl, H2SO4, CH3COOH.
Studying the crystalline conformational behavior of chitosan in the form of an acetate salt indicates the existence of four hydrated and one anhydrous forms [8].
The amine group confers polyelectrolytic properties to chitosan. Studying chitosan with molecular weight MW=200000 (according to light scattering), degree of deacetylation of 0.8 and crystallinity of 75% by G. I. Tyupenko et al. [9] has shown that due to the large number of amino groups in the macromolecules, chitosan is well soluble in aqueous solutions of diluted acids, where it behaves as a typical poly-cation. Like other poly-cations, it can interact with negatively charged poly-anions, thus forming interpolyelectrolytic complexes [6]. The interpolyelectrolytic reactions between oppositely charged poly-ions in aqueous solutions are cooperative and completely reversible.
The presence of two hydroxylic and primary amino groups in chitosan expands the possibility of its modification. Particularly interesting in synthesizing chitosan derivatives is the obtaining of directed substituted compounds (synthesis of O – or N – derivatives).
Chitosan has been acetylized with acetic anhydride [10]. Samples with various degrees of acetylation have been obtained. It has been shown that increasing the degree of acetylation decreases the characteristic and dynamic viscosity by 40% and 66%, respectively. Conformation of the molecule, and hence its characteristic viscosity are markedly dependent on the ionic strength and pH of the solution. At a given ionic strength and acidic pH values of the solution, the elongation of the molecule increases along with the degree of acetylation, due to the tendency to form hydrogen bonds between the OH group in the sixth position of one hexose link with carbonyl in the acetamide group of the next link. The solubility of chitosan samples with pH value close to neutral (pH 5 to 8) increases with the degree of acetylation [11]. At pH 6.0, chitosan samples solubility increased 2-3 times, with the degree of acetylation increasing from 0.15 to 0.75.
According to the studies [12], the availability of amino groups in the polysaccharide chain does not virtually change in case of chitosan immobilization of up to 3% of its content in the sample of the sorbent. Higher chitosan content in the sample is impractical, since with increasing the thickness of layer, hindered diffusion starts playing the major role, and prevents the penetration of ions or molecules of the sorbate into the granules.
Practical use of silicas in biomedicine has certain requirements to the relevant dosage forms of the drugs.
Today, the problem of the criteria for evaluation of sorbents for various purposes used in medical practice (hemo-and enterosorption, application therapy) is widely discussed [13]. The following requirements have been put forward for the use of sorbents:
- high adsorption capacity against a broad spectrum of toxic substances and microorganisms;
- chemical inertness;
- mechanical strength;
- non-toxicity;
- ease of sterilization;
- stability of properties during storage;
- absence of harmful effect to the viable tissues of wounds, white blood cells, macrophages;
- absence of allergic and pyrogenic reactions.
In our opinion, silica sorbents meet these requirements best. The existing problems are associated with selecting the types of silica, development of the technology for optimal modification of their surface, and the methods of adsorption immobilization of biologically active substances.
Fine silica, aerosil, is promising for the use in medicine for therapeutic and preventive purposes [14]. The Nomenclature Review Committee of the Pharmacological Committee of the Ministry of Health of the Russian Federation has assigned to aerosil the symbolic name “oxyl”. This silica is a light white powder, consisting of particles with particle size between 4 and 40 microns. Aerosil has been included into several editions of pharmacopoeias – Hungarian, Danish, and Austrian ones. In Russia, aerosil is used in pharmacy as a filler in manufacturing pills, and as a thickener in liniments.
Unlike coal adsorbents, aerosil can manifest pexic properties by binding large amounts of water, protein and microorganisms. So, aerosil A-300, when added to water, forms fairly firm gel containing aerosil and water at the ratio of 1:15. This sorbent is capable of binding up to 800 mg of protein per 1 g of the carrier in an aqueous medium fast enough. These properties of aerosil are of interest, since many biologically active substances are of protein nature (antibodies, antigens, enzymes, receptors, bacterial endo – and exotoxins). These properties of aerosil can be successfully used for thickening biological fluids, dehydration, deproteinization, immunosorbtion, bacterial and immunodiagnostics, preservation of cells and tissues, immobilization of enzymes and microorganisms, and for treating pyoinflammatory processes [14].
High degree of chemical purity creates a real basis for using aerosil as a substance carrier for obtaining medical preparations of prolonged action.
Thus, we have determined that in the experimental research in order to obtain organic-silica sorbents, it is expedient to use siliceous sorbent aerosil A-380 as the source material, which has developed specific surface, chemical inertness, high sorption capacity, and biological compatibility. Chitosan is a promising biopolymer for obtaining composite sorbents [15]. It is biologically compatible, biodegradable, has natural origin, and a known chemical structure. The presence of the hydroxyl and primary amino groups makes it possible to modify the surface, and confers polyelectrolytic properties to it.
The most efficient way of obtaining synthesis of organic-silica and element-containing sorbents is the method of forming porous structure of the silica matrix in the presence of polymers, and the method of molecular layering, which allows to obtain highly stable and specific for some biological substances sorption materials with standard composition and structure.
Methods
Sorbents of standard composition and structure that possess high sorption activity have been obtained by the method of forming porous structure of the silica in the presence of polymers.
Aerosil A-380 (GOST 14922-77, c.p.) with the content of base material SiO2 of 99.9% was used for the research. The silica sorbent is a product of high-temperature vapor-phase hydrolysis of tetrachloride silicon in the stream of oxygen with subsequent condensation in water vapors. Aerosil is a non-porous silica, where the form of particles is close to spherical. Samples of the “silochrom” C-120 macro-porous silica and aerosil gel obtained from aerosil A-380 were used as the objects for structure comparison.
The characteristics of silica sorbents are shown in Table 1.
|
Name of the sorbent |
Content |
Specific surface area S, m2/g |
Pore size d, nm |
Pore volume V, cm3/g |
Structure type |
|
|
SiO2, % |
-OH of groups, mEq./g |
|||||
|
Aerosil А-380 |
99.9 |
1.95 |
380 |
– |
– |
Amorphous |
|
Silochrom С-120 |
99.8 |
1.27 |
125 |
32 |
1.42 |
Amorphoglobular |
|
Aerosil gel |
99.8 |
1.43 |
138 |
38 |
1.40 |
Amorphoglobular |
The following substances were used as sorbent modifiers : chitosan, which is the fully de-acetylized product – poly [(1-4) – 2 – amino – 2 – deoxy – β – D – glucose] obtained at the Voikov Factory (Moscow) with molecular weight Mw = 200,000, degree of de-acetylation of 0.8, and the crystallinity of 75%; sulphochitosan; γ – aminopropyltriethoxysilane, which is a colourless liquid soluble in ethanol, toluene and acetone with boiling point at 379 K, density of 0.949 g/cm3; with the lysozyme preparation being produced at the Olaynensk Chemical Factory.
This work is aimed at obtaining sorbents with specified composition and certain biological action.
Sorbents of standard composition and structure that possess high sorption activity have been obtained by the method of forming porous structure of the carrier substance in the presence of polymers.
The non-porous amorphous silica structure, aerosil A-380 was used for the structural units forming silica frame. The 3% solution of chitosan in 3% acetic acid, and the 3% solution of sulpho-chitosan in 3% acetic acid were used as the organic components of synthesis.
The formula of obtaining organic silica sorbents include 6 stages, which are shown in Figure 2.
 |
Figure 2: The formula for obtaining organic silica sorbents |
Stages 1-3 characterize the process of obtaining composite adsorbents on the basis of forming porous structure of the silica matrix in the presence of the components of synthesis.
In stage 1, hydrogel is formed from aerosil due to the processes of condensation, involving the silanol groups of silica. In stage 2 (maturing and syneresis of the hydrogel), dehydration processes occur, being accompanied by decreasing the volume of the hydrogel, and its compaction. In stage 3, the hydrogel turns into xerogel during heat treatment, while its volume is reduced more than 8-16 times due to the action of the capillary forces. The degree of gel frame contraction depends on the ratio of the capillary forces and on
the strength of gel frame opposing them. Stages 4 through 6 show the final process of composite sorbents synthesis, which ensures separation of finely dispersed fractions and obtaining sterile sorption material.The mechanism of forming porous organic silica sorbents may be represented as a complex process accompanied by formation of corpuscular structures from non-porous aerosil core silica particles, and by including organic polymers (chitosan, sulpho-chitosan) due to multi-point adsorption in the cation exchange centers on the silica carrier surface. Thus, the structure of composite adsorbents is presented by a corpuscular system that consists of silica particles coated with organic polymers associated with each other in a single spatial frame. The size of the particles determines the magnitude of the specific surface, density of particles packing, pore volume and radius. In case of corpuscular structure of composite adsorbents, pores and voids between the corpuscles are packed in various ways. The considered mechanism of forming composite sorbents is consistent with the data from literature about obtaining composite sorbents for affine chromatography and for carriers of immobilized enzymes [1].The chitosan-silica sorbents were obtained in accordance with the synthesis formula as follows: 3% solution of chitosan in 3% acetic acid was added to 5 g of aerosil A-380. The obtained product was subjected to gelation from 3 to 24 hours at 295К; the hydrogel had been preliminarily placed into porcelain cups. Next, the sorbent was dried to xerogel for 2 hours at 368-378К, and crushed; then fractions with the particle size of 70-150 microns were separated by sieving. The sulpho-chitosan-silica composite sorbents have also been obtained in accordance with this method.
Results
The structural characteristics of sorbents have been studied. The specific surface area was determined by the method based on low-temperature nitrogen adsorption, the total pore volume and pore diameter were determined by the method of mercury injection.
Table 2 shows the characteristics of chitosan-silica composite sorbents obtained by forming the porous structure of the silica matrix in the presence of organic polymeAnalysis of the data shown in the table indicates that increasing the amount of chitosan in the composition of the sorbent 2-4 times causes a decrease in the specific surface area by 36-43% and an increase in the total pore volume. The found regularity can be explained by the influence of the nature of the intermicellar fluid on formation of hydrogel structure, and a stabilizing effect of the organic polymer, chitosan, which is manifested by the opposition to the process associated with increasing corpuscular particles in the structure of the obtained composite sorbents.
Table 2: Characteristics of sorbents depending on the amount of chitosan used in the synthesis
|
Sample name |
Mass ratio of the synthesis components |
Time of gel formation, h |
Specific surface area, m2/g |
Pore volume, cm3/g |
Pore diameter, nm |
|
|
SiO2 |
chitosan |
|||||
CSCS1.5 |
5 |
1.5 |
3 |
80 |
1.64 |
36 |
|
CSCS0.75 |
5 |
0.75 |
3 |
125 |
1.5 |
32 |
|
CSCS0.39 |
5 |
0.39 |
3 |
140 |
1.2 |
28 |
The microstructure of the synthesized composite adsorbents was studies at the IMZ – T 3000 scanning electronic microscope in comparison with known silica materials, such as macroporous glass, silochrom, and C – 120 hydrothermal silochrom.
It has been found that C – 120 silochrom has uniform globular structure with the size of fused silicon oxide particles about 20 nm. The topography of macroporous glass surface is presented a spongy structure.
A sample of composite chitosan-silica sorbent has modified globular structure combined with formed fusions of irregular shape. Unlike the structure of macroporous glass, extensive areas of amorphous formations with spongy structure are found in samples of composite adsorbents. The microstructure of the composite sorbent that contains sulpho-chitosan as organic polymer is similar to the microstructure of the chitosan-silica sorbent described above.
This regularity is confirmed by the diffused reflectance spectra of composite chitosan-silica sorbents, as compared to aerosil A-380 and the aerosil gel based on this aerosil.
The presence of organic polymer, chitosan, in composite sorbents results in a sharp increase in absorption intensity. Moreover, with increasing amount of chitosan in the composition of sorbents, a natural increase in absorption is observed. The obtained results about the regularities of changes in the spectral characteristics of the composite chitosan-silica sorbents are comparable with the diffuse reflectance spectrum of the initial chitosan polymer. The spectra of diffuse reflectance of sulpho-chitosan-silica sorbents have been studied. The results show that when the number of sulpho-chitosan in the composition of the sorbents had been increased, natural increase in absorption was observed in the spectral range between 280 and 680 nm.
The obtained data for the spectra of diffuse reflection can be used for optimizing the conditions of composite sorbents synthesis, their identification, and quantitative definition of organic polymer content in the composition of an adsorption material.The IR spectra of the obtained composite chitosan-silica sorbents have been studied in the range of wave numbers of 4,000-500 cm-1. In the IR spectrum of aerosil gel based on aerosil A-380, the absorption bands of 3,750 cm-1 and 3,680 cm-1 correspond to unbound and bound hydroxyl groups, respectively. In the IR spectrum of chitosan organic polymer, characteristic bands 1,430 cm-1 and 2,800 cm-1 are observed, as well as 3,365-3,445 cm-1, which correspond to –NH2 groups, and characteristic bands of amide groups – 3,265 and 3,100 cm-1. In the IR spectrum, the composite chitosan-silica sorbent, as compared to aerosil, shows a decrease in the absorption intensity band of 3,750 cm-1, and at the same time, appearance of the absorption bands in the region of 1,430 cm-1, 2,800 cm-1 and 3,365 – 3,445 cm-1 is observed, which is characteristic of amine and amide groups of chitosan.
Conclusion
Thus, we have performed the research of the method of composite organic silica sorbents synthesis by forming a porous structure of silica in the presence of chitosan and sulpho-chitosan with standard structural characteristics; the specific surface area varies between 80-140 m2/g, pore volume – between 1.2 and 1.64 cm3/g.
The chemical composition of composite organic silica sorbents, and the surface microstructure, as compared to the data of IR spectroscopy and diffuse reflectance spectroscopy have been studied.
It has been found that the surface microstructure of composite sorbents is represented by a combination of areas of amorphous formations with spongy structure.
The presence of amino groups on the surface determines polyelectrolytic properties of sorption materials.
Further research will be focused on enzymes immobilization on the obtained composite chitosan-silica matrices.
The authors express their gratitude to the employees of the Stavropol State Agrarian University who assisted in performing the scientific research
References
- Brykalov, A.V. Production of biopreparations based on the methods of affine absorption and immobilization Thesis of PhD of Chemistry, St. Petersburg, 1993, 330.
- Brykalov, A.V. Silica and activated carbons-based adsorbents in biotechnology and medicine. Proceedings of the Conference of chemists of North Caucasus, Nalchik, 1991, 185-186.
- Brykalov, A.V., Kabankova A.N. Directed synthesis of carbon sorbents for medical purposes. Chemistry of highly organized substances and scientific principles of nanotechnology. Proceedings of the III International Conference, St. Petersburg State University,Saint Petersburg, 2001, 171-172.
- Dergunova, E.V., Shipulya A. N. New opportunities of using chitosan in various areas of biotechnology. Issues of ecology and plant protection in agriculture: Proceedings of the 73d Scientific and Practical Conference, Stavropol, 2009, 78-81
- Zhogolev, K.D., Nikitin V.Y., Tsygan V.N. Prospects of clinical use of immunomodulatory drugs based on chitosan. Medical Immunology, 2001, 3, 2,316-317.
- Ivanov, V.A., Smirnov I.V., Rayevski V.M. Obtaining chitosan from bees. New achievements in studying chitin and chitosan: Proceedings of the 6th International Conference, VNIRO, Moscow, 2001, 24-25.
- Ogawa, K., Inukai S. X-Ray diffraction study of sulfuric, nitric and halogen acid salts of chitosan. Carbohydr. Res., 1987, 160, 425-433.DOI:10.1016/0008-6215(87)80328-2
CrossRef - Yamamoto, A., Kawada J., Yui T., Ogawa K. Conformational behavior of chitosan in the acetate salt: An X-ray study. Biosci. Biotech. Biochem., 1997, 61, 1230-1232.
CrossRef
- Tyupenko, G.M., Skorikova E.E., Zezin A.B. Electrophoresis of chitosan in treating periodontal diseases. New achievements in studying chitin and chitosan: Proceedings of the 6th International Conference, VNIRO, Moscow, 2001, 241-247.
- Ilyin, A.V., Varlamov V.P., Acetylation of LMW water-soluble chitosan. New achievements in studying chitin and chitosan: Proceedings of the 6th International Conference, VNIRO, Moscow, 2001, 280-283.
- Wang, W., Xu D. Viscosity and flow properties of concentrated solutions of chitosam with different degrees of deacetylation. Int. J. Biol. Macromol., 1994.
CrossRef
- Tertykh, V.A., Yanushpolsky V.V. Chemical reactions with participation of silica surface. The Ukr. Chem. Journal, 1972, 38, 8, 774-779.
- Petrova, V.A., Tarasenko G.A., Berezhnova L.V., Limitations of using chitosan for medical and prophylactic purposes. New achievements in studying chitin and chitosan: Proceedings of the 6th International Conference, VNIRO, Moscow, 2001, 220-222.
- Chuyko, A.A., Bogomaz V.I., Lutsyk N.B. Revisiting substantiation of the possibility to use silica – aerosil for therapeutic and preventive purposes. Medical purpose adsorbents and the mechanisms of their therapeutic action. Proceedings of the IV Republican Conference, Donetsk MI, Donetsk, 1988, 39-40.
- Levshin, M.V., Shipulya A.N. Silica sorbents and the possibility of their use. Youth, science, creativity-2009. Proceedings of the Scientific and Practical Conference, Stavropol,2009, 101-103.

This work is licensed under a Creative Commons Attribution 4.0 International License.









