Synthesis and DFT Calculations of Dinuclear complex of Co2+, Ni2+ and Cu2+ with macrocyclic Schiff base Ligands
Eid Abd Al-Razaq*,1,2, Nabeel Buttrus3, Enas H. Mohammed4 and Abdel Aziz Jbarah5
1Chemistry Department, College of Science, University of Al-Hussein Bin Talal, Ma’an, Jorda 2Chemistry Department, College of Science, Islamic University in Madinah, Al-Madinah Al-Munawrah, Kingdome of Saudi Arabia 3Chemistry Department, College of Science, University of Mosul, Mosul, Iraq 4Department of Science, Basic Education College, University of Mosul, Mosul, Iraq. 5Chemistry Department, College of Science, Islamic University in Madinah, Al-Madinah Al-Munawrah, Kingdome of Saudi Arabia corresponding author E-mail : eidalzooby@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/320114
Article Received on :
Article Accepted on :
Article Published : 02 Mar 2016
The 20-, 34- and 36-membered macrocyclic of bis (dithiodimine) Schiff base ligands, BisN,N’(dithiocarbonyl) terephthliden (L1), Bis-N,N’[1,3(o-aminophenylthio)-propane] terephthylidene (L2) and Bis-N,N-[1,4(o-amino phenylthio)-butane] terephthylidene (L3), have been prepared by a (2+2) condensation of terephthaldehyde with dithiooxamide or 1,3-bis-(o-aminophnylthio) propane,or1,4-bis-(o-aminophenylethio)-butane. Air stable dinuclear complexes of Co2+, Ni2+ and Cu2+ were obtained from reaction of metal salts with L1, L2 and L3 in tetrahydrofuran. Ligands consist of two S2N2 donor sites coordinated with the metal ions. Also, adducts of the cobalt complex with 1,4-phenelyene diamine was also presented. CHN elemental analysis, metal content, molar conductivity,magnetic measurements, proton nuclear magnetic resonance, UV-visible and infrared spectral studies have characterized the complexes and adducts. In addition to this, the DFT i.e. Density Functional Theoretical calculations has been used for supporting experimental data. This process used B3LYP functional method. It is the method which has been introduced due to Yang, Parr and Lee. This method is comprised of 3-parameter functional because of the presence of Axel Becke. It further incorporates the basis set of Los Alamos National Laboratory 2 double-zeta (LANL2DZ). Furthermore, the calculation associated with the molecule’s vibrational frequencies was calculated with the help of optimized geometry. Tetrahedral and square planar geometry around Co2+,Ni2+ and Cu2+have been deduced on the basis of magnetic and spectra studies.
KEYWORDS:Dinuclear complexes, Schiff base; macrocyclicbis (dithiodimine); density functional theory (DFT)
Download this article as:| Copy the following to cite this article: Al-Razaq E.A, Buttrus N, Mohammed E. H , Jbara A. A. Synthesis and DFT Calculations of Dinuclear complex of Co2+, Ni2+ and Cu2+ with macrocyclic Schiff base Ligands. Orient J Chem 2016;32(1). |
| Copy the following to cite this URL: Al-Razaq E.A, Buttrus N, Mohammed E. H , Jbara A. A.Synthesis and DFT Calculations of Dinuclear complex of Co2+, Ni2+ and Cu2+ with macrocyclic Schiff base Ligands. Orient J Chem 2016;32(1). Available from: http://www.orientjchem.org/?p=14505 |
Introduction
Recent research in the field of modern supra-molecular chemistry is emphasizing on the study and designing of well arranged and well organized metal containing macro-cycle complexes1.
Such complexes are of interest not merely for their uncommon structures; rather, also for their unique functional characteristics such as magnetism and luminescence redox activity2-4. The conformational rigid, Ni2+ based cationic molecular square planar [Ni(HL)]44+ as well as Cu2+ based natural molecular rectangle [Cu2Cl2L]2 have been attained with the help of self-assembly from new stiff pentadentate, N4S, ligand bis [phenyl(2-pyridyl) methanone) thioscarbazone, [H2L]. Analyses of crystal structures have depicted that the tetranuclear Ni2+ cation [Ni(HL)]44+ is situated over the center or inversion, which contains four atoms of nickel within the square’s corners, having Ni….Ni isca 4.8A° as the edge length. It is further Observed that center of each metal is being octa-hedrally coordinated via pyridine nitrogen, carbazone nitrogen atoms and sulfur atoms, which are associated with 2 perpendicular HLÉ Ligands5.
Chandra and Kumar6, reported the preparation of the complexes of Mn2+ and Cr3 +, having the general formulae of [Cr(L)X2 ]x and [Mn(L)X2],from N2O2,N2S2 and N4 donor macrocyclic ligands; 2,3diphenyle, 1,4-diaza, 7,10-dioxo, 5, 6:11,12-dibenzo[e,k]-cyclododeca, 1,3diene[N2O4 ] ane, 2,3-diphenyl, 1,4-diaza, 7,10-dithia, 5,6:11,12dibenzo[e,k]-cyclo dodeca-1,3 diene [N2S2 ]ane, and 2,3-diphenyl, 1,4,7,10-tetraaza, 5,6:11,12dibenzo[e,k]-cyclododeca 1,3-diene[n4] ane. The complexes have been characterized with the help of molar conductance measurements, elemental analysis, mass, proton nuclear magnetic resonance (1H-NMR), spectral method (infrared (IR)), electronic spectra and electron paramagnetic resonance (EPR) and magnetic measurements.
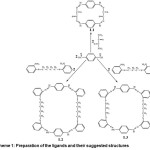 |
Scheme 1: Preparation of the ligands and their suggested structures Click here to View scheme |
Amononuclear Cu+ complex [Cu(Ca2dapt)] ClO4 and two dinuclear Cu+ complexes [{Cu(pph3)
(X)}2(Ca2dapte)], where X = Br and I, of new Ca2daote, tetradentate N2S2 donor Schiff base ligand, have been created, where Ca2dapte = N,N’-bis(transcinnamaldehyde)-1,2-di(0-iminophenyl thioethane. Characterization of these compounds has been carried out with the help of IR, elemental analyses, H-NMR and UV-visible spectroscopy. Moreover, the method of single crystal X-ray diffraction has been used for determining the crystal structures of all the mentioned7.
New complexes of 2-aminoethyl pendantarmed schiff base macrocyclic, [ML]2+,where M=Mn2+, Mg2+, Zn2+, and Cd2+, are produced with the help of M2+ template [1+1] cyclocondensation of 2,6diacetyl pyridine, having newly branched hexamine, N,N,N’,N’-tetrakis (2-aminoethyl)-2,2-dimethyl propane-1,3-diamine. Furthermore, the X-ray diffraction data has been deployed for determining the crystal structures of [MgL]+2 and [MnL]+2 were determined by X-ray diffraction data8.
The Schiff base bis[4-hydroxycuomarin3-yl]-1N,5N thiocarbohydrazone, has been produced through carrying out the reaction of thiocarbohydrazide with 4-hydroxy coumarine-3carbaldehyde, in the molar ration of 1:2, respectively. It is further identified that the binuclear complexes of ligand as well as ligand itself, having Cr3+, Mn2+, Fe2+, Cd2+, Ni2+, Cu2+ and Zn2+ ions are also characterized with the help of 1HMR and mass spectrometry, elemental analysis, magnetic measurements as well as electronic and infrared spectra9.
In view of these results and in continuation of our studies on Pd, Pt and Cu metal complexes with macrocyclic Schiff-bases10, 11, it can be reported that the preparation of new, 20, 34, and 36 membered macrocyclicbis(dithiodimine) Schiff base ligands of L3, L1, and L2 derived from terephthaldehyde and dithiooxamide or 1,3-bis-(o-aminophnylthio)propane, or 1,4-bis-(o-aminophenylethio)-butaneScheme 1, as well as their complexes and adduct with Co2+, Ni2+ and Cu2+ metal ions. The potential application of the synthesized complexes is antibacterial against some bacteria and many industrial and natural applications.
Additionally, we may report the results of the calculations of DFT for vibrational frequencies as well as geometry optimization of the synthesized molecules with the help of LANL2DZ basis set and B3LYP functional.
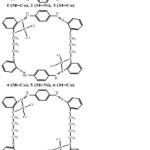 |
Figure 1: The Suggested Structures for the Synthesized Complexes Click here to View figure |
Experimental
All of the chemicals have found to be analytical reagent grade. Solvents were dried over an appropriate reagent and distilled prior to use. The chloride salts of Co2+, Ni2+ and Cu2+ were obtained from BDH. The compounds 1,3-bis-(o-aminophenylthio) butane and 1,4-bis(o-aminophenylethio) butane were developed with the help of the published method, Geary12.
Preparation of the compounds
Preparation of ligand (L1), bis-N,N’(dithiocarbonyl) terephthliden
Table 1: Physical properties, Elemental analysis, UV-Vis. and 1H-NMR measurements of the free ligands
|
Ligand Color m.p. (0C) |
%C |
%H |
%N |
%S |
1HNMRsignal in | UV/Vis |
| DMSO-d6(ppm)
|
(cm-1) | |||||
|
L1 Dark 200-204 |
55.3 |
2.64 |
13.02 |
29.22 |
6.5(m*), |
30487 , |
|
orange |
(55.04)a |
(2.75)a |
(12.89)a |
(29.34)a |
8.0-10.2(m*) |
32467 |
|
L2 Dark 124-126 |
70.99 |
5.9 |
7.02 |
15.85 |
1.8-1.9(m*), |
3246, |
|
green |
2.9-3.1(m*), |
36764 |
||||
|
(71.64)a
|
(5.47)a
|
(6.96)a
|
(15.92)a
|
7.1-7.2(m*) 7.3-8.32(m*), 8.6-8.7(m*) |
||
|
L3 Greenish 100-102 |
70.93 |
5.11 |
6.97 |
16.03 |
31055, | |
|
yellow |
(71.13)a |
(5.15)a |
(7.21)a |
(16.49)a |
32679 |
a: Calculated value, m*= multiplet
Table 2: Complex’s elemental analysis, molar conduct ance and magnetic data
|
Colour |
m.p. |
%C |
%H |
%N |
%M Meff |
Λohm-1 | Yield | |
|
No.
|
(0C)
|
|
|
|
(
|
B.M)
|
.cm-2. mol-1 |
( % ) |
|
1 Dark brown |
120d |
34.39 34.49a |
1.69 1.75a |
8.00 8.05a |
16.8316.93a |
4.20 |
12 |
85 |
|
2 Dark green |
210d |
34.43 34.52a |
1.70 1.73a |
8.01 8.05a |
16.5816.87a |
3.90 |
9 |
83 |
|
3 Dark kaki |
172d |
34.00 34.05a |
1.67 1.70a |
7.90 7.95a |
18.21 18.02a |
2.06 |
9 |
70 |
|
4 Dark green |
178d |
54.10 54.15a |
4.10 4.14a |
5.21 5.26a |
11.32 11.08a |
4.50 |
22 |
77 |
|
5 Green
|
182 184 |
54.16 54.19a |
4.11 4.13a |
5.92 6.22a
|
11.71 11.04a |
3.71 |
36 |
60 |
|
6 Olive |
110d |
53.61 53.68a |
4.09 4.10a |
5.20 5.22a |
11.73 11.84a |
1.80 |
19 |
75 |
|
7 Dark green
|
227 229 |
53.19 53.29a |
3.81 3.86a |
5.38 5.41a |
11.53 11.38a |
2.15 |
60 |
75 |
|
8 Dark kaki |
269d |
53.30 53.34a |
3.80 3.86a |
5.39 5.42a |
11.58 11.38a |
Dia. |
20 |
67 |
|
9 Dark green
|
244 246 |
52.80 52.83a |
3.78 3.83a |
5.42 5.36a |
11.96 12.16a |
2.13 |
27 |
73 |
a: Calculated value;d: decomposition temperature; M: corresponding transition metal
A solution consists of 0.001 mol of dithiooxamide in 15cm3 of methanol was added drop wise to a solution that consists of 0.002 mol of terephth-aldehyde in 10cm3 of absolute ethanol at the rate of approximately one drop every 20 seconds. After addition was completed, the mixture was boiled
under reflux for 2 hours. The orange solid product, which was formed on cooling, has been filtered off. It is further washed with diethyl ether and ethanol. Afterwards, it has been dried through the vacuum for a couple of hours.
Preparation of (L2), N,N’-bis-{1,4-(oaminophenylthiol)-butane}tere- phthylidene)
A solution consists of 0.002 mol of terephthaldehyde in 10 cm3, having absolute ethanol. This solution has been added gradually in the boiling solution that consists of 0.002 mol of 1,4-Bis(o-aminophenylthio)-butane in 20 cm3 of tetrahydrofuran. This final mixture was then boiled under reflux for the period of 2 hours. The solid product was then filtered off. The filtered solution was then washed with diethylether and ethanol. The washed solution is then dried with the help of vacuum for a couple of hours.
Preparation of (L3), N,N’-bis-{1,3-(oaminophenylthio)-propane}tere- phthylidene
A solution consists of 0.002 mol of terephthaldehyde in 10 cm3. This solution has been included gently within the boiling solution that consists of 0.002 mol of 1,3-Bis-(o-minophenylthio)-propane in 15 cm3 of tetrahydrofurane. The final mixture was boiled in the presence of reflux for the period of 2 hours. Then, the solid product, which formed on cooling, has been filtered off. The filtered solution is then washed with diethylethere and ethanol and it has been dried with the help of vacuum for numerous hours.
Metal Complex’s Preparation
A general technique has been deployed in order to prepare the binuclear complexes through carrying out the reaction between the macrocyclic Schiff base ligands and metal salts in 1:2, L:M molar ratio. The solution of the metal salt was heated inside the water bath for ensuring absolute dissolution of all the present metal salts.
A solution of ligand that consists of 0.001 mol of (L1), or (L2) or (L3) in 10 cm3 tetrahydrofuran was added gradually to the solution of the metal salt. The reaction mixture was refluxed for 3 hours with constant stirring. The solid precipitated of the complexes were filtered off, washed several times with ethanol, and then washed with diethylether and dried with the help of vacuum over CaCl2.
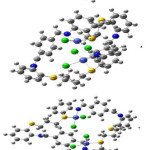 |
Figure 2 Click here to View figure |
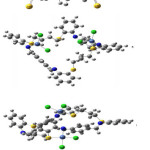 |
Figure 3 Click here to View figure |
according to b3LYP/LANL2Dz level theory accordance with LANL2Dz/b3LYP level of theory
Physical Measurements
IR spectra were recorded on Bruker Tensor
27 (FITR) spectrophotometer in the range of 4000250 cm-1 with the help of the technique of CsI disc. In addition to this, 1cm quartz cell has been used in order to record electronic spectra over Shimadzu UV160 spectrophotometer. It has been carried out for complex’s 0.001 M solution, present in DMF (i.e. dimethylformaamide). Furthermore, the complex’s 0.001 M solution present in DMF has been used for carrying out conductivity measurements. These measurements have been carried out at ambient temperatures and it also made use of conductivity
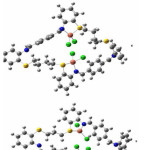 |
Figure 4 Click here to View figure |
meter 4070 Jenway. Additionally, Faraday’s method has been deployed for carrying out magnetic measurements at the temperature of 25°C over the Solid state through the use of the instrument of Bruker BM6. Pye Unicam (SPG) atomic absorption spectrophotometer has been used for carrying out metal content analyses. The Micro analytical center located in Al-Albait university of Jordan has been used for carrying out the microanalyses of hydrogen, sulfur, carbon and nitrogen. Varian 300 MHz NMR spectrophotometer are used for carrying out ligand’s 1 H-NMR spectra.
Computational Details
Gaussian03 suite of programs has been deployed for carrying out all calculations and evaluations of the study13. Complete optimization of accordance with LANL2Dz/b3LYP level of theory all complex’s geometries at B3LYP14-16 level has been carried out through the use of Los Alamos National Laboratory 2 double-zeta basis set (i.e. LANL2DZ)1719. Such basis set is considered as the commonly deployed ECP (i.e. Effective Core Potential) type of basis set. Thus, it is deployed commonly along with the methods of density functional for carrying out the study of TM (Transition Metals) containing systems. ECP has represented chemically inactive major electrons. As a result of these electrons, the cost of computation has been reduced because of the fact that the increment in cost is resulted from ~N4, in which N is the number of such electrons which are treated explicitly. MOLDEN program and Gaussian, Inc. provided GaussView 3.0. It has been deployed to inspect the output as well as input files produced by Gaussian0320. This program is deployed for the purpose of structure modification, post processing and preprocessing analyses of frequencies, forces and structures. It has been further found that for positively identifying most stable and steady structure, the frequency and the minima analysis were carried out for ever stationary point. Such analysis allowed in ensuring that all of the minima did not contain any kind of imaginary frequency within the vibrational mode computations. It is due to the fact that transition states encompass single imaginary frequencies over the potential energy surface.
Results and Discussion
The new Schiff base type macrocycle ligands, i.e. L2, L3 and L1, with two S2N2 donor sites as two linked compartments were prepared by the [2+2] condensation of terephthaldehyde with 1,3-Bis(o-aminophenylthio)- propane or 1,4bis(o-aminophenylthio)-butan in ethanol. These reactions proceed in high yields (>80%), and the characterization of the products was carried out with the help of 1H-NMR, UV-Vis, spectroscopy and elemental analysis. Results are listed in table 1.
1H-NMR data for ligand (L1) showed that H%CP%Nproton appears at 7.4 ppm as multiplet signals. Moreover, the multiplet signals at 8.0-8.19 ppm are related to the protons of phenyl groups. While ligand (L2) showed the CH2 proton at 1.8-1.9 ppm, with the signals of CH2-S appeared at 2.9-3.1 ppm (m) and H-C=N proton as multiplet at 7.1-7.2 ppm and the signal at 7.3-8.32 ppm for phenyl protons. These analyses have found to be in good conformity along with the formula proposed for L1, L2 and L3, as displayed in Scheme 1. The reactions of these macrocyclic Schiff base ligands with the salts of MCl2.6H2O, M= Co, Ni and CuCl2.2H2O produce complexes of general formula [M2Cl4Ln], n= 1,2, or 3, with 2:1 molar ratio of M:L.
The physical and chemical properties of prepared complexes have been depicted in the form of table 2. The complexes are all amorphous, air-stable and soluble in most organic solvents except DMF and DMSO. 10-3M of DMF solution of the complexes has the molar conductance in the
Table 3: Calculated and experimental infrared absorption (cm-1) data for all of the compounds
|
Compound |
|
|
band assignment (cm-1) |
|||
|
|
ʋ (C=N) |
ʋ(C=S) |
ʋ(C-S) |
ʋ (M-N) |
ʋ (M-S) |
ʋ (M-Cl) |
|
L1 |
1600 |
836 |
– |
|||
|
1627a |
838a |
|||||
|
L2 |
1606 |
765 |
– |
|||
|
1639b |
766a |
|||||
|
L3 |
1637 |
727 |
– |
|||
|
1638a |
733a |
|||||
|
Complex 1 |
1574 |
816 |
470 |
404 |
310 |
|
|
1582a |
817a |
470a |
406a |
313a |
||
|
Complex2 |
1575 |
816 |
475 |
381 |
283 |
|
|
1532a |
841a |
488a |
364a |
289a |
||
|
Complex3 |
1572 |
815 |
475 |
365 |
305 |
|
|
1530a |
811a |
472a |
365a |
308a |
||
|
Complex4 |
1652 |
700 |
477 |
402 |
287 |
|
|
1639a |
705a |
479a |
403a |
285a |
||
|
Complex5 |
1651 |
710 |
503 |
382 |
320 |
|
|
1643a |
710a |
501a |
381a |
320a |
||
|
Complex6 |
1614 |
718 |
500 |
400 |
281 |
|
|
1627a |
721a |
499a |
400a |
284a |
||
|
Complex7 |
1625 |
674 |
413 |
381 |
320 |
|
|
1623a |
678a |
419a |
388a |
316a |
||
|
Complex8 |
1626 |
671 |
430 |
350 |
280 |
|
|
1631a |
669a |
435a |
351a |
279a |
||
|
Complex9 |
1620 |
689 |
432 |
391 |
305 |
|
|
1618a |
693a |
429a |
391a |
307a |
||
a: Calculated value according to B3LYP/LANL2DZ level
8 to 60 ohm-1cm-2mol-1 range, which is indicating a non-electrolyte nature12. It appeared to be constant and lasting along with assumed the stoichiometry, which was considered for the complexes based on its analytical data. It is further identified that the complex’s analytical data, as illustrated in table 1, are in a good conformity with the proposed formula, as displayed in figure 1.
Table 3 has listed down the most significant diagnostic characteristics of the calculated as well as recorded infrared spectra of synthesized complexes and the ligands. Allocation of determined vibrational modes, which are depicted in table 2, has been carried out through different sources of literature21-24. It is found that the L1 ligand’s infrared spectrum demonstrates sharp bands of 836 cm-1 and 1600 cm-1. These bands have been allocated to ʋ(C=S) and (C=N), respectively. In complexation, apparent differences have been observed between the complexes 1, 2 and 3 and free L1 ligand’s infrared spectra.
Table 4: Selected bond angles and bond distances (Å) of optimized structure of 1, 4 and 7 in accordance with LANL2Dz /b3LYP level of theory
bond Lengths (Å) of complex 1 bond Lengths (Å) of complex 4 bond Lengths (Å) of complex 7
|
Co(44)-Cl(46) |
2.1375 |
Cl(96)-Co(105) |
2.2323 |
Cl(98)-Co(99) |
2.2122 |
|
Co(44)-Cl(45) |
2.1594 |
Cl(95)-Co(105) |
2.2750 |
Cl(96)-Co(99) |
2.0776 |
|
Co(41)-Cl(43) |
2.1607 |
Cl(94)-Co(106) |
2.1631 |
Cl(95)-Co(100) |
2.2124 |
|
Co(41)-Cl(42) |
2.1052 |
Cl(93)-Co(106) |
2.2126 |
Cl(94)-Co(100) |
2.2250 |
|
N(40)-Co(44) |
1.8565 |
N(78)-Co(105) |
1.8549 |
N(79)-Co(100) |
1.8871 |
|
N(15)-Co(41) |
1.8639 |
N(34)-Co(106) |
1.9290 |
S(60)-Co(100) |
2.2178 |
|
S(24)-Co(44) |
2.1593 |
S(60)-Co(105) |
2.2488 |
S(37)-Co(99) |
2.2709 |
|
S(21)-Co(41) |
2.1992 |
S(37)-Co(106) |
2.2678 |
N(34)-Co(99) |
1.8814 |
Angels (°) of complex 1 Angels (°) of complex 4 Angels (°) of complex 7
|
Cl(46)-Co(44)-Cl(45) |
76.6790 |
Cl(94)-Co(106)-Cl(93) |
85.2789 |
Cl(95)-Co(100)-Cl(94) |
88.5156 |
|
Cl(46)-Co(44)-N(40) |
177.9092 |
Cl(94)-Co(106)-S(37) |
93.6052 |
Cl(95)-Co(100)-N(79) |
90.0063 |
|
Cl(46)-Co(44)-S(24) |
103.3372 |
Cl(94)-Co(106)-N(34) |
173.2601 |
Cl(95)-Co(100)-S(60) |
164.8361 |
|
Cl(45)-Co(44)-N(40) |
102.3861 |
Cl(93)-Co(106)-S(37) |
164.6347 |
Cl(94)-Co(100)-N(79) |
170.1739 |
|
Cl(45)-Co(44)-S(24) |
178.9599 |
Cl(93)-Co(106)-N(34) |
89.0561 |
Cl(94)-Co(100)-S(60) |
89.9333 |
|
N(40)-Co(44)-S(24) |
77.6323 |
S(37)-Co(106)-N(34) |
90.8899 |
N(79)-Co(100)-S(60) |
93.9919 |
|
Cl(43)-Co(41)-Cl(42) |
77.0505 |
Cl(96)-Co(105)-Cl(95) |
86.5497 |
Cl(98)-Co(99)-Cl(96) |
85.5736 |
|
Cl(43)-Co(41)-S(21) |
171.6496 |
Cl(96)-Co(105)-N(78) |
170.6101 |
Cl(98)-Co(99)-S(37) |
161.0497 |
|
Cl(43)-Co(41)-N(15) |
104.0191 |
Cl(96)-Co(105)-S(60) |
90.5867 |
Cl(98)-Co(99)-N(34) |
94.4459 |
|
Cl(42)-Co(41)-S(21) |
104.7376 |
Cl(95)-Co(105)-N(78) |
94.9985 |
Cl(96)-Co(99)-S(37) |
92.6833 |
|
Cl(42)-Co(41)-N(15) |
176.3511 |
Cl(95)-Co(105)-S(60) |
167.4939 |
Cl(96)-Co(99)-N(34) |
177.3902 |
|
S(21)-Co(41)-N(15) |
74.7183 |
N(78)-Co(105)-S(60) |
89.8078 |
S(37)-Co(99)-N(34) |
88.1418 |
The ʋ (C=S) band is shifted to lower region by about 25-28 cm-1 in the complexes of 1, 2 and 3. In addition to this, the ʋ (C=N) band has been transferred to low values, i.e. 20 to 21 cm-1, for similar complexes. Such type of shifting has suggested a complex formation by means of the co-ordination through S and N atoms of L1 ligand [25]. The infrared spectra of L2 and L3, also, showed notable differences from those for the 4, 5, 6, 7, 8 and 9 complexes. The ʋ (C=N) band appeared at 1606 and 1637 cm-1 in L2 and L3, respectively. This band is shifted to higher values with 8 to 46 cm-1 upon complexation in the complexes of 4, 5 and 6. While, in the complexes of 7, 8 and 9, the band has been transferred to the lower region by 12 to 17 cm-1. These results supported coordination through azomethine [26] in the complexes of 4, 5, 6, 7, 8 and 9. Also, the ʋ (C-S) band was identified at low values in 4, 5, 6, 7, 8 and 9 complexes as compared to those of free ligands, i.e. L1 and L2. These shifts are supporting, also, a complex formation via co-ordination through S atom.
Furthermore, the IR spectra of the metal complexes have shown new bands at 350 to 404 and 430 to 503 cm-1, which have been assigned to ʋ (M-S) and ʋ (M-N) [4, 27, 28]. Moreover, the complex’s IR spectra have shown another new band in the region of 280 to 320 cm-1, which may well be due to ʋ (M-Cl)29.
When the calculating values of vibrational frequency are compared with the values of experimental, then it is indicated that majority of the calculated values of vibrational frequency are almost same as the values of the experiment. However, the largest error occurred at ʋ (C=N) band, with the largest deviation being 43 cm-1. Such deviations have found to be pertinent to the truth that all of the theoretical calculations have been carried out for an isolated molecule within the gaseous phase. On the other hand, the results of experiment are for single molecule within the solid state. It is found that within the solid phase, the intermolecular interactions exist. Such interactions make a diversion or distraction of electron clouds between the atoms that participated in the interaction. These diversions in electron clouds between atoms result in a shift in the bands that corresponds to these atoms.
The values of magnetic moments of the Co2+ complexes 1,4 and 7 are 4.20, 4.50 and 2.15 B.M., respectively, and the electronic spectrum of the these complexes have shown broad bands at 14970 to 16835 cm-1 which, are due to 4A2→2Eg transition and another band at 31250 and 36764 cm-1 which corresponds to charge transfer, these values suggested a square planer geometry around the complexes of Co2+. Such an experimental data has been acquired with the help of magnetic moments and electronic spectra values of 1, 4 and 7 complexes,
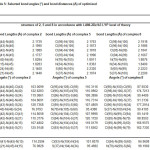 |
Table 5: Selected bond angles (°) and bond distances (Å) of optimized
|
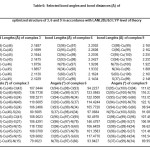 |
Table 6: Selected bond angles and bond distances (Å) of Click here to View table |
complexes which appear to be in effective conformity with the compound’s estimated geometries with the help of LANL2DZ/ B3LYP level of theory (as depicted in table 4 and figure 2). The estimated geometries assist in confirming the bonding which is present between S and N. It further helped in developing the environment of square planar about the central metal atom. However, the increment in the magnetic moment values, having the complexes of 1 and 4, are due to ferromagnetism and coupling between the two cobalt atoms.
The Ni2+ complexes 2 and 5 showed magnetic moments of 3.90 and 3.71 B.M. respectively. These values agree with high spin configuration and indicate presence of tetrahedral environment around nickel ion [30]. The electronic spectra of Ni2+ complexes have shown bands at 16168 and 16835 cm-1, which corresponds to the transition of 2T2(F)→2E(P) (ύ3) in tetrahedral environment. The diamagnetic nature in complex 8, together with the bands associated with electronic spectral, indicate a square planar geometry around the Ni2+ ion. The appearance of the band at 25252 ( )cm-1 has been allocated to 1A1g→1A2g transition within a square planar environment around nickel ion with D4h symmetry31. The calculated geometries of the complexes of 2, 5 and 8, in accordance with LANL2DZ/B3LYP level of theory, are displayed in figure 3. A chosen bond angles and distances of the optimized structures of the complexes of 2, 5 and 8 are mentioned in table 5. The calculated geometries of figure 3 confirm that the environment around nickel atomare tetrahedral in the case of 2 and 5 complexes, and square planar for the condition of complex 8
Magmatic moments of Cu2+ complexes
3, 6 and 9 have been found to be 2.06, 1.80 and 2.13 B.M., which corresponds to the occurrence of single unpaired electron in the complexes. All the complexes are strongly colored since they have highly intense absorption band in UV/Vis region. In these complexes, charge transfer bands are obscured on higher frequency side of the spectra. Besides these weaker bands, other bands are observed at 12345 to 14970 cm-1, which are assigned to 2T’!2E Transition
in tetrahedral environment32,33. The calculated geometries of 1, 4 and 7 complexes using the LANL2DZ/B3LYP level of theory (as shown in table 6 and figure 4) are strongly supported by the above mentioned results of magnetic moments values and electronic spectra
Conclusion
The macrocyclic Schift base ligands L1, L2 and L3 which are used in this study coordinate to the metal ions in tetradentate fashion from the two S2N2sites of the ligand forming dinuclear complexes, as shown in figure 1. All complexes and ligands are air stable and colored compounds. The Co-containing complexes adopted square planar geometries around the central metal ion. The Ni-containing complexes of 2 and 5 adopted tetrahedral geometries around Ni2+ion, while the complex 8 adopted a square planar geometry. All the Cu-complexes adopted tetrahedral geometries around copper ion. The geometries of complexes are estimated according to the physico-chemical measurements which are supported by theoretical calculations using DFT i.e. density functional theory through the inclusion of the basis set of LANL2DZ and B3LYP functional.
References
- Fujita M., chem. Soc. Rev.,1998, 127,417.
CrossRef
- Sun S.S., Carrell T.G., J. Am. chem. Soc.1999. 121, 557.
- Lahav M., Gabai R., Shipway A.N., Willner I., Chem. Cmmun.,1999, 1937.
- Colacio E., Lopes-Magana C., Mckee V., Romerosa A., J. Chem. Soc. Dalton Trans.1999, 2923.
- Cheng H., Chun ying D., Chen-jie F., Yongjiang L., Jin M.Q., J. Chem. Soc. Dalton Trns.2000, 1207.
- Chandra S., Kumar R., Trans. Metal Chem.2004, 29, 269 .
CrossRef
- Morshedi M., Amirnasr M., Slawin A.M.Z., Woollins J.D., Polyhedron,2009, 28, 167 .
CrossRef
- Mahammadi K., Saeid H.A., Lang H., Inorg. Chim. Acta,2007, 360, 579.
CrossRef
- AbouMelha K.S., Faruk H., J. Iran. Chem. Soc,2008., 5, 122.
CrossRef
- Buttrus N.H., Mohammed S.I., Mu’tah LilBuhuth wad- Dirasat,2004, 19, 87.
- Buttrus N.H., Mohammed S.I., Jordan J. appl. Scince,2006, 8, 18.
- Geary W.J., Coord. Chem. Rev.1971., 7, 8.
CrossRef
- Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Zakrzewski V.G., Montgomery J.A., Stratmann R.E., Burant J.C. Burant, Dapprich S., Millam J.M., Daniels A.D., Kudin K.N., Strain M.C. Strain, Farkas O., Tomasi J., Barone V., Cossi M., Cammi R., Mennucci B., Pomelli C., Adamo C., Clifford S., Ochterski J., Petersson G.A., Ayala P.Y., Cui Q., Morokuma K., Malick D.K., Rabuck A.D., Raghavachari K., Foresman J.B., Cioslowski J., Ortiz J.V., Stefanov B.B., Liu G., Liashenko A., Piskorz P., Komaromi I., Gomperts R., Martin R.L., Fox D.J., Keith T., Al-Laham M.A., Peng C. Y., Nanayakkara A., Gonzalez C., Challacombe M., Gill P.M.W., Johnson B., Chen W., Wong M.W., Andres J.L., Gonzalez C., HeadGordon M., Replogle E.S., Pople J.A., Gaussian,,2003 Inc., Pittsburgh.
- Lee C., Yang W., Parr R.G., Phys. Rev.1988, B37, 785.
CrossRef - Becke A.D., J. Chem. Phys.1993, 98, 5648.
CrossRef
- Becke A.D., J. Chem. Phys.1996, 104, 1040.
CrossRef
- Hay P.J., Wadt W. R., J. Chem. Phys.1985, 82 , 270.
CrossRef
- WadtW.R.. Hay P.J., J. Chem. Phys1985., 82, 284.
CrossRef
- Hay P.J., Wadt W.R., J. Chem. Phys.1985, 82, 299.
CrossRef
- Schaftenaar G., Noordik J.H., J. Comput.Aided Mol. Des.2000.,14, 123.
CrossRef
- Nakamoto K., Infrared and Raman spectra of Inorganic and Coordination Compounds,1986, 4 th ed., Wiley, New Yourk.
- Socrates G., Infrared Characteristic Group frequencies,1980, John Wiley & Sons, Ltd., New Yourk.
- Lin-Vien D., Colthup N.B., Fateley W.G., Grasselli J.G., The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules,1991, Academic Press, Inc, New York.
- Dollish F.R., Fateley W.G., Bentley F.F., Characteristic Raman Frequencies of Organic Compounds,1974, Wiley, New York.
- Sharma V.K., Sirvastava S., Sirvastava A., Polish J. Chem.2006, 80, 387.
- Arora K., Sharma D.P., Pathak M., Oriental 30. J.Chem.1999,15, 331.
- AL-Allaf T.A.K., Mustafa I.A., Al-Muktar S.E., Trans. Met. Chem.1993, 17, 579.
- AL-Allaf T.A.K., Sheet A.Z.M., Polyhedron, 1995, 14, 239.
- Wei G., Lawrance G.A., Richens D.T., Hambley T.W., Turner P., J. Chem. Soc. Dalton Trans.1998, 633.
- Buttrus N.H., Al.Ramadane O.M., JassimeZ.U.Int.J.Chem.2006,23, 344.
- Smith D.W., Coord. Chem. Rev.1976, 21,93.
CrossRef - Keinan S., Avnin D., J. chem Soc. Dalton Trans.2001, 941.
CrossRef
- Sherifa R., Arsha A., George L., Mohanan K., Hubert I. And Selwin R.j., Oriental journal of chemistry, 2015, 31(4), 1949-1960

This work is licensed under a Creative Commons Attribution 4.0 International License.









