Fabrication a composite electrode based on MWCNT/Zeolite for potentiometric determination of Cr3+
Zahra Heidari, Mahboubeh Masrournia*, and Razea Sannavii Khoshnood
Department of Chemistry, Faculty of Sciences, Mashhad Branch, Islamic Azad University, Mashhad, Iran
Corresponding author Email: mah.masrour@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/320171
Article Received on :
Article Accepted on :
Article Published : 27 Jan 2016
A nanocomposite based on carbon paste electrode fabricated, by using zeolite as ion carrier .Functional multi-wall carbon nanotube was used for improvement of electrode response. The optimal composition of CPE was built from 65% graphite powder, 20 wt% paraffin oil, 5% ion carrier (zeolite), 10% MWCNTs. Electrode showed Nernstian response 19.88 (±0.2)mVdecade−1 in the measuring range 10−7–10−2 mol L−1 and showed detection limit of 6.3×10-7 M. The sensor was successfully used for potentiometric titration Cr3+ with EDTA. The carbon paste electrode showed fast response time, good selectivity and applicable in the wide pH range of 3 -10.
KEYWORDS:sensor; ion selective electrode; multi-walled carbon nanotubes; potentiometry; zeolite
Download this article as:| Copy the following to cite this article: Heidari Z, Masrournia M, Khoshnood R. S. Fabrication a composite electrode based on MWCNT/Zeolite for potentiometric determination of Cr3+. Orient J Chem 2016;32(1). |
| Copy the following to cite this URL: Heidari Z, Masrournia M, Khoshnood R. S. Fabrication a composite electrode based on MWCNT/Zeolite for potentiometric determination of Cr3+. Orient J Chem 2016;32(1). Available from: http://www.orientjchem.org/?p=13721 |
Introduction
In the first decade of the nineteenth century, chromium used as a component of paints, but now its main application is (85%) for the manufacture of stainless steel and electroplating with chromium and the remainder used in chemical industries, refractory and foundry industries. With development of use of chromium compounds in various industries like leather, paint, coating metals and steel industry as well as its entering to the environment through waste factories, toxic effects have been observed on human health. [1-4].
Many techniques have been used for determination of chromium, such as ion chromatography, Flame Atomic Absorption Spectrometry, Inductively Coupled Plasma Spectrography, X-Ray Fluorescence Spectrometry, Emission Spectrography, Neutron Activation Analysis, Colorimetry, Polarography but Potentiometric method, is an electrochemical zmethod that measured the potential of the electrode system. As well as, it used to measure compounds by ion-selective electrode. Potentiometry has several advantages compared with other methods, such as, speed, ease of preparation, relatively fast response, simple instrumentation, wide linear range, reasonable selectivity, low cost [5-12].
Carbon paste electrodes, are one of the latest of the ion-selective electrodes, are consideration of, because of improvements of some factors, such as lower detection limits, the low ohmic resistance, renewability, stable response, no need for internal solution. This kind of sensor consists of a combination of ionophore, paraffin oil, graphite powder and sometimes a matter of modifier [13-19].
Multi-wall carbon nanotube is one of the most active studied material in the manufacture of carbon paste electrodes because of its unique structure and physical properties such as, Ultra-light weights, high mechanical strengths, high electrical conductivities, high thermal conductivities, metallic or semi-metallic behaviors and high surface areas. Combination of the unique properties of carbon nanotubes into materials with high potential was caused their various applications. They improve dynamic range and reduce response time [20-32].
Zeolites are crystalline and hydrate aluminasilicates with tetrahedral structure that make up from an endless three-dimensional network from tetrahedral SiO44-.and AlO45- that are connected together with oxygen atoms. Zeolites as Natural resin have properties removal of anionic pollutant and cationic exchange. After identifying unique physicochemical properties of zeolites, they attracted the attention of many researchers. Structure and chemical compound of natural zeolites and their modified form are potential sources of various researches. They are used as ion exchanger, catalyst and influence in adsorption and desorption of ions. Zeolites are attention due to the adsorption properties and porous structure and applied in the manufacture of sensors. Zeolite apply in different industries because of having cation exchange, molecular sieve, acidity and catalytic properties. The most Natural zeolites include clinoptilolite, Mordenite, phillipsite, analcime and the synthetic zeolite include X, Y, F, W, LA. Zeolite ion exchange properties are dependent upon the chemical structure of the zeolite, and change with structure and nature of the zeolite. Ion exchange capacity of the cation is dependent on the number of locations interchangeable. Cavities in zeolites make selective absorption of a specific ion or molecule in the presence of the other. They are abundant and cheap materials [33-35].
In this article, we built a nano-composite carbon paste electrode for determination of Cr3+ and used in potentiometry titration Cr3+ with EDTA.
Experimental
Chemicals
Graphite powder with a <50µm particle size (Merck) and paraffin oil (Aldrich) was used for the preparation of the carbon paste electrode. The multi-walled carbon nanotubes (MWCNTs) with 10-40 nm diameters, 1-25 µm length was purchased from Research Institute of Petroleum Industry (Iran).The nitrate salts of the cations were purchased from Merck Co. Deionized water was used for preparing all of the solutions. The natural Clinoptilolite zeolite was obtained from IRAN (FERDOWS CITY) result of elemental analysis of a natural Clinoptilolite zeolite show (SiO2 63.37%, MnO 0.02%, Al2O3 10.71%, CaO 1.69%, Na2O 4.09%, P2O5 0.05%, MgO 1.20%, Fe2O3 2.15%, K2O 2.00%, SO3 0.53%, TiO2 0.34%, LOI 12.87%.
Instruments
The electrochemical cell contained, a calomel (Azar electrode) as a refrence electrode and the chromium (III) sensor as the indicator electrode .The Schematic figure is as follows:
Carbon paste electrode | sample solution | Cl–(4M) │Hg2Cl2(s) │Hg (l) │Pt
After EMF is calculated, calibration curve is drawn by plotting potential, versus the logarithm of the concentration of the chromium ion.
Functionalization of nanotubes
The amount of the carbon nanotube with a certain amount of nitric acid and sulfuric acid was refluxed for 4 hours at 120 to 140 ° C. Materials produced was filtered by the Millipore filter and vacuum pumps and was washed with distilled water until complete neutralization of the leach solution. After drying it, using the IR spectrum was assessed the created functional groups.
Carbon paste electrode preparation
Desired amount of Zeolite, graphite powder, paraffin oil and functionalized nanotubes were thoroughly mixed and then transferred into a glass tube with i.d. 5mm and a height of 3cm. After the mixture homogenized, to avoid the formation of air gaps, the paste was packed carefully in to the tube. This can prevent an increased in the electrical resistance of the electrode .Then Electrical contact was made by inserting a copper wire in to the opposite end. The working surface of the electrode was polished by using soft abrasive paper.Then, the electrode was conditioned for 24h by being soaked in a 1.0×10-3 mol L-1 of Cr (NO3)3 solution [36-39].
Results and discussion
Potential response of the electrode
To study the selectivity behavior of designed electrode, clinoptilolite zeolite was employed as a sensing element in preparation of some carbon paste electrodes. Zeolites have regular channels and holes that have been filled by cations and water molecular. Existing cations were mobile, and can replace with other cations. Also, existing cations in the pores of zeolite can interact with some polar molecular and attract them in its external surface. Existing water molecular in zeolite network can go out of network, continuously and reversibility. After preparing of carbon paste electrode, the selectivity of the designed electrode was tested against the some cations. Electrodes were used for solution of 1×10-1 M to 1×10-7 M of the nitrate salt of the cations. Potential was measured and plotted against the logarithm of the concentration of the ions. Results are shown in Table 1. According to data, Cr3+ shows a good Nernst response. As a result, electrode is optimized for Cr3+
Table 1: The slope and r2 for plot of potential, versus, the logarithm of the concentration of some cations.
|
Mn6+ |
co2+ |
cu2+ |
Al3+ |
As3+ |
Fe3+ |
Ni2+ |
Cd2+ |
Cr3+ |
M |
|
8.80 |
27.48 |
26.22 |
15.65 |
17.00 |
17.77 |
27.37 |
34.00 |
20.14 |
slope(mVdecad-1) |
|
0.9980 |
0.9950 |
0.9920 |
0.9960 |
0.9940 |
0.9920 |
0.9960 |
0.9960 |
0.9960 |
R2 |
Optimization of CPEs
Ion carrier is a key element in the sensor, which has main role in determining of the selectivity of the membrane. It binds with certain ion. In this paper, we used Clinoptilolite zeolite as an ion carrier Clinoptilolite is a natural zeolite and has high ion exchange property, and its abundant resources are attentional in our country. High ion exchange capacity of Clinoptilolite cause that can remove heavy ions from contaminated water.
Also we used MWCNT-COOH as modifier in CPE. We know, that MWCNT cause to increase conductivity of the sensor and transduction of the chemical signal to electrical signal. As a result, dynamic working range and response time of the sensor improved, and the potential response of the sensor improves to Nernstian values. As well as, reaction acid with frame and unsymmetrical end of nanotube cause to open its end and was created functional group in breakage location on wall, that this method cause to increase solubility of nanotube and purification nanotube from amorphous carbon. By the way the toxicity of nanotubes are reduced. As a result of a more nanotube sit on surface of electrode and more analyte is attracted towards surface of electrode and are provided stronger interaction with the analyte. An important method for the detection of functionalized MWCNT is FT-IR spectrum. As can be seen, in Fig 1, the spectrum of nanotubes haven’t any specific functional group except C=C absorption band But in the absorption spectrum of functional nanotube, absorption of hydroxyl group is seen in 3000-3500 cm-1 and absorption of carbonyl group is seen in 1650-1800 cm-1.
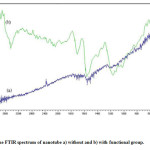 |
Figure 1: The FTIR spectrum of nanotube a) without and b) with functional group |
After we optimized amount of ion carrier and MWCNT with one variable at the time method. Results are shown at Table 2.
Table 2: Investigation of the optimal amount of carbon nanotube powder for carbon paste electrode.
|
R2 |
Slope(mVdecate-1) |
Zeolite(g) |
MWCNT-COOH (%) |
|
0.9846 |
10.00 |
1.0 |
5.0 |
|
0.9958 |
13.17 |
2.0 |
5.0 |
|
0.9976 |
15.74 |
3.0 |
5.0 |
|
0.9977 |
14.28 |
1.0 |
10 |
|
0.9935 |
17.00 |
2.0 |
10 |
|
0.9993 |
18.88 |
3.0 |
10 |
|
0.9866 |
23.51 |
4.0 |
15 |
|
0.9982 |
15.14 |
5.0 |
15 |
|
0.9951 |
13.17 |
6.0 |
15 |
|
0.9730 |
15.42 |
4.0 |
10 |
|
0.9930 |
19.98 |
5.0 |
10 |
|
0.9960 |
11.60 |
6.0 |
10 |
It can be seen from Table 2, that an electrode with composition of 5% zeolite, 10% MWCNT, 65% graphite powder, 20% paraffin oil was chosen as the optimum composition, showing a Nernstian slope of 19.98±0.2 mV decade-1
Calibration curve
The measurement range of an ISE is defined as the upper and lower detection limits, that in the range, response of the electrode obeys of nernst equation [38-40]. The response of the electrode with optimal combination was tested in Cr (III) ion concentration range of 1.0⤬10-1-1.0⤬10-7 M and the results are shown in Fig. 2.
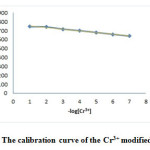 |
Figure 2: The calibration curve of the Cr3+ modified CPE. Click here to View figure |
The measuring range of nano-composite based Cr (III) CPE is between 1.0×10−7 and 1.0×10−2 mol L−1. The detection limit of the modified carbon paste electrode was 6.3 × 10−7 mol L−1 which was calculated by the extrapolating of the linear portion of the calibration curve in Fig.2.
Effect of pH
In order to evaluate the effect of pH on the potential response of the sensor, the potentials were measured for 1.0×10−3 mol L−1 of Cr3+solution with different pH values. The pH varied by addition of HNO3 or KOH. The potential was plotted versus; pH in Fig. 3. The composition of the electrode was kept constant in all experiments. The results display the potential of electrode is constant between pH (3–10) in Fig.3. Decrease in potential in above the pH value of 10.0 is because of formation of Cr3+ ion hydroxyl complexes in the solution. And decrease in potential below the pH value of 3.0 was related to the partial protonation of the zeolite [41-42].
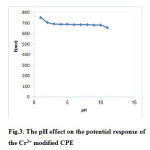 |
Figure 3: The pH effect on the potential response of the Cr3+ modified CPE Click here to View figure |
In another experiment, the values of E (mV) were examined for Cr3+ solution with a concentration of 1.0⤬10-1-1.0⤬10-7 M in the pH (2, 4, 6). The result shown, pH has no effect on potential response. But the best slope was recorded for pH (2).
Response time
Response time of an electrode is one of the important factors for every ISE. Response time of an electrode is obtained through measuring the average time after its immersion in a series of solutions of the target ions, each having a ten-fold concentration difference [43]. At this stage experiments, time response for the mentioned sensor was investigated by changing the Cr3+concentration from1.0×10−7 to 1.0×10−2 mol L−1 and the results showed that the response time of the electrode was less than 13 s (Fig 4)
Interference studies
Selectivity of electrode is ability of electrode to detect the target ion of other ions (interfering ions) and is expressed in terms of selectivity coefficients. It determined by the matched potential method (MPM). According to this method, a specified activity (concentration) of main ions (A) is added to a reference solution and the potential is measured. In a separate experiment, interfering ions (B) are successively added to an identical reference solution, until the measured potential matches the one obtained before the addition of the primary ions. The matched potential method selectivity coefficient, KMPM, is then given by the resulting main ion to interfering ion activity (concentration) ratio, KMPM = aA/aB. aA, is main ion activity(concentration). aB, is interfering ion activity (concentration) [44]. The results are shown in Table 3.
Table 3: The selectivity coefficients of various interfering cations for the Cr3+ electrode
|
K MPM |
Interference |
K MPM |
Interference |
|
10-2×4.20 10-4×4.38 10-3×4.65 1.42×10-3 10-4×2.48 10-2×3.71
|
Fe3+ K+ La3+ Mn6+ Ni2+ Al3+ |
10-4×2.69 10-4×1.20 10-3×5.39 10-3×3.50 10-3×4.98 10-3×3.79
|
Ca2+ Cd2+ Ce3+ Co2+ Cu2+ As3+ |
As can be seen, alkali, alkaline earth and transition metals don’t disturb the measurement of chromium with this electrode.
Life time
The lifetime of the electrodes, the length of time that the electrode is sensitive to changes in concentration of the target ion and slope is acceptable. The average lifetime reported for most ISEs in the range of 4 to 10 weeks. After this time slope will be reduced and the detection electrode increases .in order to investigate the lifetime of the sensor, a series of experiments were performed using 3 electrodes for 10 weeks and after 8 weeks, slight decline in the electrode potential response was observed .So life time of the sensor was estimated 8 weeks.Results are shown in Table 4.
 |
Table 4: Lifetime of Cr (III) nano-composite CPE Click here to View table |
Analytical Application
Fabricated electrode was used for detection of the end point of titration Cr3 + with EDTA. 50 ml of solution of 1.0×10-4 M Cr3+ was titrated with solution of 1.0×10-3 M EDTA. With addition of EDTA, potential values reduced, because complex of Cr3 + with EDTA formed, as a result, concentration of free Cr3 + ions in solution decreased. Potential changes around of the point of equivalent sharply, which represents behavior of sensor is successful to measure of Cr3 + in potentiometric titration .The curve is shown in Fig.5.
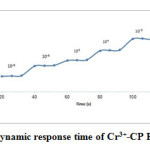 |
Figure 4: The Dynamic response time of Cr3+-CP E. Click here to View figure |
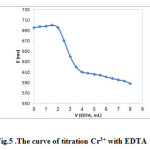 |
Figure 5: The curve of titration Cr3+ with EDTA Click here to View figure |
The new Cr3 +-selective CPE was used for determination of Cr3 +in waste water sample. The method repeated five times, and results were recorded. Then Atomic Absorption Spectroscopy (AAS) was applied to determination of Cr3 + ion concentration in waste water sample. The results were compared with the data obtained from atomic absorption spectrometry (AAS). As can be seen in Table 5, there was a satisfactory agreement between the results of the developed sensor and those obtained by AAS.
Table 5: Determination of Cr3 + in waste water.
|
Spectrophotometric method |
ISE (ppm)a |
Sample no. |
|
0.71±0.10 |
0.70±.020 |
1 |
|
2.10±0.10 |
2.20±0.30 |
2 |
|
3.51±0.10 |
3.50±0.30 |
3 |
a Results are based on three measurements
Comparison of the proposed electrode (V) with literature precedents
To compare this electrode with other chromium sensitive electrodes that reported in the literature [45-54], linear range, detection limit, operating pH range, response time and slope of these electrodes are given in Table 6. As can be understood from Table 6, the electrode in measurement of chromium ion is more successful than other reported electrodes because of having a wide measuring range the lower detection limit, fast response time, much wider pH range. Different Cations don’t interfere in measuring of Chrome with these electrode so this sensor is useful for determining of Cr3+.
Table 6: Comparison of proposed electrode with other Cr3 + selective electrode.
|
Detection limit |
pH range |
Slope |
Response time |
Concentration range |
Ref |
|
5.3×10−7 |
3.2–6.3 |
19.60 |
10s |
1.0×10−6-1.0×10−1 |
45 |
|
6.3×10−7 |
2.7–6.5 |
19.80 |
<20 |
3.0×10−6–1.0×10−2 |
46 |
|
1.6×10−7 |
4.8–6.3 |
20.51 |
∼8 |
3.2×10−7-1.0×10−1 |
47 |
|
5.8×10−7 |
2.7-6.6 |
19.70 |
10 |
1.0×10−6-1.0×10−1 |
48 |
|
2.0×10−7 |
2.2-5.0 |
19.80 |
>10 |
2.0×10−7-1.0×10−1 |
49 |
|
2.0×10−7 |
4.5-6.5 |
20.00 |
15 |
4.0×10−6-1.0×10−1 |
50 |
|
6.3×10−7 |
2.6-6.5 |
19.80 |
<20 |
3.0×10−6-1.0×10−2 |
51 |
|
8.0×10−7 |
3.0-5.5 |
19.50 |
10 |
1.66×10−6-1.0×10-2 |
52 |
|
6.3×10−7 |
1.7-6.5 |
19.80 |
20 |
1.0×10−7-1.0×10−2 |
53 |
|
1.0×10−6 |
3.5-6.5 |
19.00 |
10 |
7.0×10−6-1.0-×10-1 |
54 |
|
1.0×10−7 |
3.0-10 |
19.88 |
<13 |
1.0×10-7-1.0×10-2 | This work |
Conclusion
In the present work, we used zeolite as selective ionophore for fabrication a nanocomposite CPE for determination of Cr3+. Electrode can use well in range pH (3-10) with response time less than 13 s. The linear range for determination of Cr3+ with this electrode is 1.0×10−7 – 1.0×10−2 with nernstian slope of 19.88 and detection limit of 6.3.0×10−7. Electrode can used in the presence alkali, alkaline earth and transition metals without inconvenience.
References
- Emmanueul, S.; Adamu, I.; Ejila, A.; Ja’afaru, M.; Yabaya, A.; Habila, B. Journal of Toxicology and Environmental Health Sciences. 2014, 6, 89-98.
CrossRef - Xu, X. R.; Li, H. B.; Gu,J. D.; Li, X. Y. Environmental toxicology and chemistry. 2005, 24, 1310-1314.
CrossRef - Cheung, K.; Gu, J. D. International Biodeterioration & Biodegradation. 2007, 59, 8-15 .
CrossRef - Fernandes,H. M. Environmental Pollution. 1997, 97, 317-325.
CrossRef - Ali, T. A.; Aglan, R. F.; Mohamed, G. G.; Mourad, M. A. International Journal of Electrochemical Science. 2014, 9, 1812-1826.
- Ali, T. A.; Mohamed, G. G.; Azzam, E. M. S.; Abd-Elaal, A. A. Sensors and Actuators, B: Chemical. 2014, 191, 192-203.
CrossRef - Ali, T. A.; Mohamed,G. G.; El-Dessouky, M. M. I.; El-Ella, S. M. A.; Mohamed,R. T. F. Journal of Solution Chemistry. 2013, 42, 1336-1354.
CrossRef - Ali,T. A.; Eldidamony, A. M.; Mohamed,G. G.; Elatfy, D. M. International Journal of Electrochemical Science. 2014, 9, 2420.
- El-Hariri, T. Y. M. A.; Mousa, A. S.; Moustafa, T. F.; Farouk, S. Egyptian Journal of Petroleum. 2012, 21, 71.
- Ganjali, M. R.; Moghaddam, M. R.; Hosseini, M.; Norouzi, P. Int. J. Electrochem. Sci, 2011,6,1981-1990
- Mohammadabadi, F.; Zamani, H. A.; Joz-Yarmohammadi, F. M.; Abedi, R. Int. J. Electrochem. Sci. 2015, 10, 2791-2800.
- Frag, E. Y. Z.; Ali,T. A.; Mohamed, G. G.; Awad, Y. H. H. International Journal of Electrochemical Science.2012, 7, 4443.
- Mohamed, G. G.; Ali, T. A.; El-Shahat, M. F.; Migahed, M. A.; Al-Sabagh, A. M. Drug Testing and Analysis. 2012, 4, 1009.
CrossRef - Wang, Y.; Wei, W.; Zeng, J.; Liu, X. ; Zeng, X. Microchimica Acta. 2008, 160, 253-260.
CrossRef - Javanbakht, M.; Badiei,A.; Ganjali, M. R.; Norouzi,P.; Hasheminasab,A.; Abdouss, M. analytica chimica acta. 2007, 601, 172-182.
CrossRef - Javanbakht, M.; Ganjali, M. R.; Norouzi, P.; Badiei, A.; Hasheminasab, A.; Abdouss,M.Electroanalysi. 2007, 19, 1307-1314.
CrossRef - Goyal, R. N.; Oyama, M.; Gupta, V. K.; Singh,S. P. ; Sharma, R. A. Sensors and Actuators B: Chemical. 2008, 134, 816-821.
CrossRef - Javanbakht, M.; Eynollahi- Fard, S.; Abdouss, M.; Mohammadi, A.; Ganjali, M. R.; Norouzi, P.; Safaraliee, L. Electroanalysis, 2008, 20, 2023-2030.
CrossRef - Rezaei, B.; Damiri, S.Sensors Journal, IEEE.2008, 8, 1523-1529.
CrossRef - Siswana, M.; Ozoemena, K. I.; Nyokong, T. Sensors, 2008, 8, 5096-5105.
CrossRef - Li, G.; Xu, H.; Huang, W.; Wang, Y.; Wu, Y.; Parajuli, R.. Measurement Science and Technology. 2008, 19, 065203-065209.
CrossRef - Bianco, A.; Hoebeke, J.; Godefroy, S.; Chaloin, O.; Pantarotto, D.; Briand, J. P.; Muller, S.; Prato, M.; Partidos, C. D. Journal of the American Chemical Society. 2005, 127, 58-59.
CrossRef - Bianco, A.; Kostarelos, K.; Prato, M. Current opinion in chemical biology. 2005, 9, 674-679.
CrossRef - Klumpp, C.; Kostarelos, K.; Prato, M.; Bianco, A. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2006, 1758, 404-412.
CrossRef - Kostarelos, K.; Lacerda, L.; Pastorin, G.; Wu, W.; Wieckowski, S.; Luangsivilay, J.; Godefroy, S.; Pantarotto, D.; Briand, J. P.; Muller, S. Nature nanotechnology, 2007, 2, 108-113.
CrossRef - Lacerda, L.; Bianco, A.; Prato, M.; Kostarelos, K. Advanced drug delivery reviews 2006, 58, 1460-1470.
CrossRef - Lacerda, L.; Pastorin, G.; Wu, W.; Prato, M.; Bianco, A.; Kostarelos ,K. Advanced Functional Materials, 2006, 16, 1839-1846.
CrossRef - Pantarotto, D.; Singh, R.; McCarthy, D.; Erhardt, M.; Briand, J. P.; Prato, M.; Kostarelos, K.; Bianco, A.Angewandte Chemie. 2004, 116, 5354-5358.
CrossRef - Pastorin, G.; Wu, W.; Wieckowski, S.; Briand, J.-P.; Kostarelos, K.; Prato, M.; Bianco, A. Chemical communications.2006, 1182-1184.
CrossRef - Singh, R.; Pantarotto, D.; Lacerda, L.; Pastorin, G.; Klumpp, C.; Prato,M.; Bianco, A.; Kostarelos, K.Proceedings of the National Academy of Sciences of the United States of America. 2006, 103, 3357-3362.
CrossRef - Chou, A.; Böcking, T.; Singh, N. K.; Gooding, J. J. Chemical communications.2005, 7, 842-844.
CrossRef - Nezamzadeh-Ejhieh, A.; Mirzaeyan, E. Electrochimica Acta. 2011, 56, 7749-7757
CrossRef - Nezamzadeh-Ejhieh, A. ; Afshari, E. Microporous and Mesoporous Materials.2012, 153, 267-274
CrossRef - Nezamzadeh-Ejhieh, A.; Badri, A. Journal of Electroanalytical Chemistry. 2011, 660, 71-79
CrossRef - Ali, T. A.; Mohamed, G. G.; Al-Sabagh, A.; Migahed, M. Chinese Journal of Analytical Chemistry.2014, 42, 565 -572.
CrossRef - Ali, T. A.; Farag, A. A.; Mohammad, G. G. Journal of Industrial and Engineering Chemistry 2014, 20, 2394-2400.
CrossRef - Ali, T. A.; Eldidamony,A. M.; Mohamed, G. G.; Abdel-Rahman, M. A. Int. J. Electrochem. Sci 2014, 9, 4158-4171.
- Ganjali, M. R.; Memari, Z.; Faridbod, F.; Dinarvand, R.; Norouzi, P. Electroanalysis. 2008, 20, 2663-2670.
CrossRef - Ganjali, M. R.; Norouzi, P.; Faridbod, F.; Yousefi, M.; Naji, L.; Salavati-Niasari, M. Sensors and Actuators B: Chemical. 2007, 120, 494-499.
CrossRef - Gupta, V. K.; Jain, S.; Chandra, S. Analytica chimica acta. 2003, 486, 199-203.
CrossRef - Ganjali, M. R.; Faridbod, F.; Norouzi, P.; Adib, M. Sensors and Actuators B: Chemical. 2006, 120, 119-124.
CrossRef - Zamani, H. A.; Rajabzadeh, G.; Ganjali, M. R. Sensors and Actuators B: Chemical. 2006 , 119, 41-46.
CrossRef - Gupta, V. K.; Al Khayat, M.; Singh, A. K.; Pal, M. K. Analytica Chimica Acta 2009, 634, 36-43.
CrossRef - Zamani, H. A.; Rajabzadeh, G.; Masrornia, M.; Dejbord, A.; Ganjali, M. R.; Seifi, N. Desalination. 2009, 249, 560-565.\
CrossRef - Gholivand, M. B.; Sharifpour, F. talanta. 2003, 60, 707-713.
CrossRef - Ghaedi, M.; Shokrollahi, A.; Salimibeni, A. R.; Noshad, S. , Joybar, S. Journal of Hazardous Materials.
CrossRef
- 2010, 178, 157-163.
- Zamani, H. A.; Rajabzadeh, G.; Ganjali, M. R. Sensors and Actuators B: Chemical 2006, 119, 41-46.
CrossRef - Kumar, P.; Sharma H. K. ,Electrochimica Acta 2013, 87, 925-929.
CrossRef - Gupta, V.; Jain, A.; Kumar, P.; Agarwal, S.; Maheshwari, G. Sensors and Actuators B: Chemical. 2006, 113, 182 -186
CrossRef - Gholivand, M.; Sharifpour, F. Talanta. 2003, 60, 707-713.
CrossRef - Abbaspour, A.; Izadyar, A. Talanta. 2001, 53, 1009-1013.
CrossRef - Gholivand, M. B.; Raheedayat, F. Electroanalysis. 2004, 16, 1330-1335.
CrossRef - Sharma, R. K.; Goel, A. Analytica Chimica Acta 2005, 534, 137-142.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









