Optimizition of uranium adsorption process using TOAFMNPs as a novel adsorbent from synthetic sulfuric acid and real sample media
Nemat Mansouri1, Kamal Saberyan2* and Mohammad Noaparast3
1Department of Mineral Processing, College of Engineering, Tehran Science and Research Branch, Islamic Azad University, Tehran, Iran 2NFCRS, Nuclear Science and Technology Research Institute, P.O. Box: 11365-8486, Tehran, Iran. 3Mineral Processing Engineering Department, School of Mining Engineering, University College of Engineering, University Of Tehran, Tehran, Iran. Corresponding Author Email: Saberyan@aeoi.org.ir
DOI : http://dx.doi.org/10.13005/ojc/31.Special-Issue1.34
Article Received on :
Article Accepted on :
Article Published : 07 Nov 2015
In this study, optimization of uranium (VI) adsorption process using Triocthylamine (TOA) functionalized magnetite nanoparticles (TOAFMNPs) as a novel adsorbent from synthetic sulfuric acid and real media were investigated. Firstly, this adsorbent was synthesized and characterized. Then the synthetic sulfuric acid solutions were prepared and the effects of different variables affecting uranium adsorption from these solutions were evaluated in detail. The results revealed that the optimal conditions were achieved in pH=5, contact time of 20 min, adsorbent amount of 3 g l-1 , and U (VI) initial concentration of 10 mg l-1. Under these conditions associated elements (Al, Fe, Mg, and V)had negligible effect on uranium adsorption and maximum percentage of U (VI) ions adsorbed by the TOAFMNPS was 94.6%. The maximum adsorption capacity was evaluated as 27.5 mg g-1 at room temperature. The selective stripping of U (VI) from the loaded TOAFMNPs was obtained by using sulfuric acid. The uranium adsorption process form real sample media was investigated in optimal conditions.The real sulfuric acid solutions used in this study were obtained by leaching of ore deposit of second anomaly of Sag hand mine. At optimum condition 80.8% of U (VI) in pregnant leach solution (PLS) was adsorbed on TOAFMNPs without any interference of other metals. Based on the experiments this novel adsorbent may present potential application in the adsorption of U (VI) in these real solutions.
KEYWORDS:Uranium (VI); Adsorption; Magnetite nanoparticles; TOA; second anomaly of Saghand mine
Download this article as:| Copy the following to cite this article: Mansouri N, Saberyan K, Noaparast M. Optimizition of uranium adsorption process using TOAFMNPs as a novel adsorbent from synthetic sulfuric acid and real sample media. Orient J Chem 2015;31(Special Issue1). |
| Copy the following to cite this URL: Mansouri N, Saberyan K, Noaparast M. Optimizition of uranium adsorption process using TOAFMNPs as a novel adsorbent from synthetic sulfuric acid and real sample media. Orient J Chem 2015;31(Special Issue1). Available from: http://www.orientjchem.org/?p=12379 |
Introduction
Uranium extraction and separation has been linked to the development of new technologies to simplify the flow sheets and improve associated process economics.
The various methods are available for the extraction and adsorption of uranium from aqueous solutions. Liquid-liquid extraction, ion exchange and solid phase extraction are commonly used techniques.However, the mentioned methods are complex processes that suffer from large secondary wastes, significant chemical additives, solvent losses, complex equipment, and bulky design1, 2. Various adsorbents are being investigated for the adsorption of uranium ions from aqueous solutions such as magnetite3, natural4 and synthetic zeolites 5.
In the last decade, magnetic nanoparticles (MNPs) have been widely investigated for several applications including separation and extraction of target ions. The advantage of the MNPs is that they can be separated by external magnetic field and the magnetic particles surface can be modified to suit the application such as chemical and physical separation, remediation and mineral processing.6-12.
Recently,the magnetically assisted chemical separation (MACS) process was used as an effective alternative to conventional methods such as solvent extraction and ion exchange. In this method, MNPs have functionalized with a solvent extractant and applied as adsorbent for the adsorption of target ions from the aqueous solutions. This technique has advantages such as very simplicity, easily handling and economically cheapest technique when compared with other analytical and separation techniques. Recent studies have shown that the MACS process to be an effectiveness method for the separation of transuranics13-15, cesium16, strontium17, cobalt and nickel1.Shaibu et al. have used Cyanex 923-coated magnetic particles for recovery of Th (IV), U (VI), Am (III) and Eu (III) from synthetic nuclear waste solutions. They showed that MACS process for the adsorption of uranium has more efficiency than traditional solvent extraction2. Nunezet al. evaluated TOPO and D2EHPA coated nanoparticles as an adsorbent for efficiently separation of radionuclides such as americium and uranium from acidic solutions. These adsorbent were three orders of magnitude higher than that of traditional solvent extraction techniques13, 14.
S. Sadeghi et al. studied silica-coated magnetite nanoparticles modified with quercetin. These magnetite nanoparticles were assessed as a new solid phase sorbent for recovery of uranium from aqueous solutions. The adsorption equilibrium of uranium onto the sorbent was explained by Langmuir isotherm and maximum monolayer adsorption capacity was found 12.33 mg/g. The synthesized sorbent was applied to recovery of uranium from different water samples18.
The adsorption features of synthetic MNPs have been investigated by R. Leal and M. Yamaura19 for the removal of uranium from nitric solutions. Batch experiments were carried out to evaluate the adsorption of uranium from nitric solution (in pH= 4 and 5) onto MNPs. Equilibrium adsorption isotherm was evaluated using Freundlich and Langmuir models. The adsorption was between 40 and 80% under studied conditions.
Amides/amines are the principal classes of nitrogen based compounds which are used for the uranium extraction and separation technology. A great number of amides20-25and amines26-31 was used for the uranium extraction from various sources.
The extraction of uranium from aqueous sulfuric acid by tri-octylamine (TOA) in benzene was investigated as a function of different experimental parameters27.Theextraction of uranium from acid heap leach liquor was studied using tertiary amines (tri‑n‑octylamine) as extractants because of their high selectivity and efficiency28.
Alamine 336 was used as extractant for uranium extraction from sulfate solutions32-34. Other works concluded that nitrogen based extractants such as amines are good class of organic complexing extractants for the extraction of uranium and separation from its leach solutions35.
The objective of this study was to assess the feasibility of TOAFMNPs using as adsorbent for uranium adsorption from synthetic sulfuric acid and real media. Based on literatures, little research efforts have been made on investigation of uranium removal or adsorption by MNPs from leach liquor. In this study, the preparation of adsorbent (TOAFMNPs) was described and the obtained products were characterized by different methods and used in the adsorption of U (IV) from the synthetic and real solutions. To achieve the maximum adsorption efficiency, various parameters were studied and optimized. The adsorption isotherms and kinetics were also investigated.
Exprimentals
Materials
All chemicals including FeCl2.4H2O, FeCl3.6H2O, NaOH and HCl with analytical grade were supplied from Merck Company (Germany) and were used without further purification.
The commercial grade amine based extractant Triocthylamine (Specific gravity: 0.891 g ml-1) from NFCRSwas used as received without purification.
Stock solutions of U (VI), Al3+, Fe2+, Mg2+ and V (V) ions were prepared by dissolving appropriate amounts of UO2 (NO3)2.6H2O, Al(NO3)3.9H2O, FeSO4.7H2O, MgO4S.7H2O and V2O5, respectively in deionized water at the required initial concentrations. Other concentrations prepared from stock solution by dilution and fresh solutions were used for each experiment. The initial pH of the working solutions was adjusted by the addition of dilute H2SO4 or NaOH solutions.
Apparatus
Synthesized magnetic nanoparticles were characterized by XRD measurements (STADI-MP, STOE Company, with monochromatized Cu Kα radiation). Fourier transform infrared (FT-IR) spectroscopy (Bruker Vector 22 model, Germany) was used for the studying of functional groups at a resolution of 4 cm-1. The spectra were scanned at wavenumber range of 400-4000 cm-1.Synthesized nanoparticles were dispersed in diethyl ether by ultrasonic cleaner (model clean 01). The pH of solutions was measured using pH meter (model 827 Metrohm). Determination of the metal ions concentration in the aqueous solutions was performed by inductively coupled plasma optical emission spectrometry (ICP-OES) (Perkin-Elmer 2000 DV model).
Preparation of the adsorbent (TOAFMNPs)
According to our previous work36, MNPs were prepared by normal co-precipitation method with iron salts (FeCl2.4H2O, FeCl3.6H2O) and polyethylene glycol (PEG)[reaction (1)].
Fe2+ + 2Fe3+ + 8OH– + PEG → Fe3O4 + 4H2O + PEG (1)
For preparation of the adsorbent 300 mg of MNPs were dispersed in 10 mL of diethyl ether by an ultrasonic bath for 15min. The appropriate amount of TOA (for example 0.04 mL of TOA for TOA/MNPs (w/w) 10%) in diethyl ether was then added to it and left until diethyl ether evaporated. Afterwards the stock of TOA-functionalized MNPs with concentration 3 g L-1 were prepared by addition of 100 mL of deionized water. The adsorbent nanoparticles in aqueous solution were black emulsion with stability for more than one month and easily separated from the solution in a few seconds, under an external magnetic field. Observations demonstrated easy and fast separation of the adsorbent after the adsorption experiments.
TEM and XRD studies
The particle size and morphology of the MNPs and prepared adsorbent were determined by transmission electronic microscopy (TEM) and powder XRD.
Fig.1 shows the typical TEM images of the MNPs and TOAFMNPs. TEM analysis of the nanoparticles indicated that both pure MNPs (Fig.1 a,b) and TOAFMNPs (Fig.1 c, d) were approximately spherical in nature with sizes ranging from 10 to 15 nm for MNPs and 20 to 25 nm for TOAFMNPs.
The synthesized nanoparticles were characterized by XRD method. According to Fig.2, the powder XRD pattern of the synthesized MNPs was close to the pattern for crystalline magnetite Fe3O4. Using the most intense peak in MNPs XRD pattern, the particles size of 10 nm was estimated by the Debye-Scherer equation37, which was consistent with the results obtained from the TEM image.
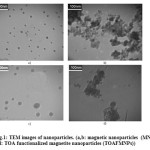 |
Figure 1: TEM images of nanoparticles. (a,b: magnetic nanoparticles (MNPs). c,d: TOA functionalized magnetite nanoparticles (TOAFMNPs)) Click here to View figure |
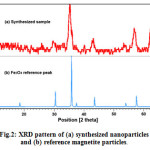 |
Figure 2: XRD pattern of (a) synthesized nanoparticles and (b) reference magnetite particles. Click here to View figure |
FT-IR spectrum of the adsorbent
FT-IR spectroscopy analysis was performed to study the surface composition of the modified MNPs. The functional groups of amine-functionalized MNPs were identified using this technique.
This fact that amino-group has been attached onto theFe3O4 nanoparticles was proven by comparison of FT-IR spectra of the coated and uncoated Fe3O4 nanoparticles shown in Fig.3. It can be seen that, compared with theuncoated sample, the coated Fe3O4 nanoparticles possess absorption bands in the region of 2919 cm-1 due to the asymmetric stretching of the C–H2 groups, two weak bands around 2919 cm-1 due to symmetric stretching of C–H2 and asymmetric stretching of C–H3, a band at 1085 cm-1due to C–N stretching vibrations. All of these reveal the existence of TOA. In addition, the absorption bands centered around 3451 and 1634 cm-1assigned to the vibration of remainder H2O in the samples.
The characteristic absorption bands of the Fe-O bond of bulk Fe3O4 were in 570 and 375 cm-1. Hence, the strong IR band at 575 cm-1 is characteristic vibration of Fe-O in Fe3O4. Figure 4 shows the structural formula of TOA.
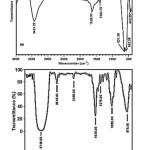 |
Figure 3: FT-IR spectrum of (top) Fe3O4 nanoparticles, (bottom) TOAFMNPs. |
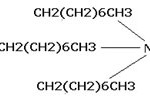 |
Figure 4: Structural formula of TOA. |
Batch Experiments
A batch method was applied to investigate the adsorption of metal ions from aqueous solutions.30 mg of adsorbent was added to 50 mL of synthetic sulfuric acid media then this mixture was stirred at 300 rpm by mechanical stirrer.
The synthetic sulfuric acid media were prepared by dilution of the standard solution stock of U (VI) (100 mg l-1), Al3+ (200 mg l-1), Mg2+ (1000 mg l-1),Fe2+ (200 mg l-1), V (VI) (200 mg l-1), with deionized water.Initial concentration of these ions in synthetic experiment was simulated according to real leach liquor composition.
The effect of variable parameters on adsorption process was determined by equilibrating the adsorption mixture containing accurate amount of adsorbent and uranyl ions in different conditions.
Preliminary experiments showed that the mixture was stirred for 20 min to ensure adsorption equilibration. Under an external magnetic, adsorbent was allowed for 1 min to settle and completely gathered to one side of the solution and the clear supernatant was directly decanted and uranium in the aqueous phases was determined by ICP‑OES.
The amount of adsorbed U (VI) was determined from the difference between the U (VI) concentration in the aqueous before and after the adsorption. The percent adsorption (%) of U (VI) from solution was calculated as follows.
Adsorption (%) = (Co -Ce)v/m (3) Where C0 and Ceare concentration of U (VI) at initial and equilibrium time (mg l-1), respectively. The adsorption capacity (qe, mg g-1) of U(VI) was achieved by the following equation.
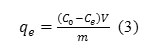
Where V is the solution volume (mL) and m is the mass of adsorbent (g).
Results and Discussion
Experiments in Synthetic Sulfuric Acid Media
For optimization of the U (VI) adsorption process several experiments have been performed and results are shown in this section.
Effect of TOA/MNPs weight ratio (w/w) and adsorbent amount
In this experiment for preparation of 100 mL stock of adsorbent with TOA/MNPs weight ratio (w/w) 10%, according to section 2.3, 0.04 mL of TOA (specific gravity of TOA: 0.891 mg l-1) was added to diethyl ether containing 300 mg of MNPs.
Adsorption of U(VI) on TOAMNPs was studied by varying the TOA/MNPs weight ratio between 10 to 100% while keeping the other parameters constant (stirring time 20 min, initial U (VI) Concentration 10 mg l-1, pH=3 and adsorbent amount 15 mg).
According to Fig.5, adsorption (%) at TOA/MNPs weight ratio (w/w) >10% was observed to be less because of MNPs were stacked to each other and were inhibited from dispersion in the solution during adsorption and decreased adsorption values. The maximum adsorption, 60.2 %, was achieved at 10% of TOA/MNPs weight ratio.
To optimizing the adsorbent amount for 10 mg l-1 U (VI) adsorption, the adsorbent effect was studied in the range of 15 to 60 mg in 5 to 15 mL from stock of adsorbent with concentration 3 g l-1. The results of experiments are shown in Fig.6. It was found that the percentage ofU (VI) adsorption increased from 60.2% to 95.4% by increasing adsorbent amount until the value of 30 mg.
After this amount the adsorption remained almost constant (data not presented). This was attributed to the fact that adsorption was optimum for the specific U (VI) initial concentration. Therefore, by increasing the adsorbent amount, the effective surface area was increased up to the amount of 60 mg. Therefore, for the rest of the experiments an adsorbent amount of 30 mg was used, to exclude entirely a possible effect caused from the adsorbent amount.
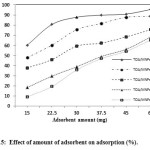 |
Figure 5: Effect of amount of adsorbent on adsorption (%). Click here to View figure |
Effect of pH
The first step of the adsorption process development is to know the optimum acidic condition or pH condition to quantitatively adsorption the targeted metal. In this study, the experiment related to pH effect was carried out in the range of 1 to 7 at U (VI) concentration: 10 mg l-1, amount of adsorbent: 30, 45 and 60 mg, TOA/MNPs (w/w): 10% and time of 20 min.
The results (Fig.7) showed that the adsorbent influence on the percentage adsorption was increased with the increase of pH up to 5 whereas, it was reversed (decreased) at higher pH range like 5 to 7.
This results show that the adsorption ability of adsorbent for U (VI) is high in near neutral conditions and low in strong acidic conditions. The maximum adsorption value for U (VI) onto TOAFMNPs was about 86.7%, 91.1% and 95.6% for 30 mg, 45 mg and 60 mg of adsorbent, respectively. Therefore, it was found that the adsorption efficiency of U (VI) is high in pH 5 and accordingly, pH 5 was considered as the optimum value and which has also been chosen for the prior U (VI) extraction by TOAFMNPs.
The pH of real solution is about 1.5 to 2. In this experiment maximum adsorption (81.6%) in this pH was obtained with using 60 mg of TOAFMNPs.
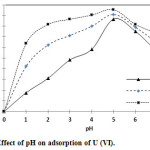 |
Figure 6: Effect of pH on adsorption of U (VI). |
It is well known that the species of uranium are strongly dependent on pH value. Fig.7 (a, b)exposes that the free uranyl ion UO22+ is the dominant species in the acidic pH range up to 5. With increasing pH the uranyl ion becomes more hydrolyzed and forms a series of hydrolyzed U (VI) species such as UO2(OH)+, [(UO2)2(OH)2]2+ and [(UO2)3(OH)5] + are formed. 38
 |
Figure 7: Fraction amounts of species uranium at different pH.39 Click here to View figure |
It can be induced that these species are exchanged at the functional groups on the TOAFMNPs. It is also well known that the surface of TOAFMNPs negatively charged hence it can absorb positively charged uranyl ion species or other hydrolyzed species through electrostatic interaction. At low pH, the H+ ions present at acidic solution which competes with positively charged uranium species for the adsorption sites, resulting in reduced uptake of U (VI).
In the other hand, sulfate is a relatively weak complexate for uranyl, but if sufficient amounts are present, this will influence U (VI) speciation up to approximately pH 5; UO2SO4 and UO2(SO4)2-are present in the pH range of 2-6, at pH >6 the hydrolyzed uranyl complexes become the dominant species in solution (Figure 7b).
Sulfate can clearly reduce the U (VI) uptake by TOAFMNPs at acidic conditions. This may be explained by the competition between uranyl and sulfate ions for the surface sites of TOAFMNPs, or by the formation of uranyl-sulfate complexes in the aqueous phase at pH >6, sulfate has a small impact on the U (VI) sorption onto TOAFMNPs due to the presence of the hydrolyzed uranyl complexes instead of the sulfate-uranyl complexes.
Kinetic study of adsorption
The adsorption kinetics governing the residence time adsorption reaction determines the solute uptake rate or efficiency of adsorption. Hence, the rate of U (VI) adsorption on TOAFMNPs was determined as a function of the initial U (VI) ions concentrations. The uptake of U (VI) ions for three initial U (VI) concentrations in the range of 5-50 minstirring time is showed in Fig.8. It was observed that lowest concentration showed the highest adsorption U (VI). The adsorption was rapid in first 10 min and then gradually attained equilibrium within 20 min (92.3% adsorption). The rapid adsorption rate has significant practical importance, which ensures high efficiency and cost-effectiveness. This equilibrium time were used in all subsequent adsorption tests.
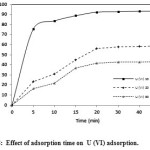 |
Figure 8: Effect of adsorption time on U (VI) adsorption. Click here to View figure |
The kinetic of adsorption has been studied to investigate the mechanism of the adsorption. Four kinetic models were tested to fit experimental data of uranium adsorption on TOAFMNPs: pseudo-first-order, pseudo-second-order, Elovich and Intra-particle diffusion model.
The results of the experimental data fitting with these models for the adsorption of U (VI) onto TOAFMNPs are shown in Table 1.
The coefficients of correlation, R2for the pseudo-second order adsorption model had a high value (≥ 0.99). In addition, equilibrium adsorption capacities from the experiment (qeexp: 15.7 mg g-1) were better closed to those determined from the pseudo-second order model (qecalc:16 mg g-1) than those from the pseudo-first order model. These facts suggest that the pseudo-second order adsorption mechanism is predominant.
Therefore, chemisorptions may be the rate determining step of the adsorption process rather than mass transfer in solution.
| Table 1: Kinetic parameters for the adsorption of U (VI) on TOAFMNPs. | ||
| Kinetic Models | Parameter | Value |
| Pseudo-first-order | K1 (min-1)qecalc (mg g-1)qeexp(mg g-1)
R21 |
0.032.8516
0.754 |
| Pseudo-second-order | K2 (g mg-1 min-1)qecalc (mg g-1)qeexp(mg g-1)
R22 |
0.0515.716
0.999 |
| Intraparticlediffusion | h (mg g-1 min)Ki (mg g-1 min0.5)C (mg g-1)
R2 |
0.0790.55311.88
0.761 |
| Elovich | α (mg g-1 min-1)β (g mg-1)R2 | 132500.3410.884 |
Adsorption isotherms
To optimize the design of an adsorption system for the adsorption of adsorbate, it is important to establish the most appropriate correlation for the equilibrium data. Several adsorption isotherm equations are available and two important isotherms are selected in this study, the Langmuir and Freundlich isotherms. The equilibrium data obtained in the present study were analyzed using the linear forms of the expressions of Langmuir and Freundlich isotherm models.
Fig.9 depicts the adsorption capacity as a function of initial U (VI) concentration. It was observed that the amount of U (VI) adsorbed on the TOAFMNPs increases with increase in initial U (VI) concentration.
This can be due to the increase in specific adsorption sites at the adsorbent surface which enhances the interaction between adsorbate and the adsorbent. This figure also shows that adsorption capacity was increased with increasing U (VI) concentration up to 33 mg l-1and more increase in U (VI) concentration has no significant effect on adsorption capacity. This can be attributed to the saturation of specific adsorption sites on TOAFMNPs. The maximum adsorption capacity for U (VI) was determined experimentally to be 27.5 mg g-1.
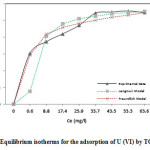 |
Figure 9: Equilibrium isotherms for the adsorption of U (VI) by TOAFMNPs Click here to View figure |
The isotherm parameters of each model were calculated by linear regression analysis and the values are given in Table 2. A comparison is also made between two isotherms plotted in Fig.9, which shows the experimental data points and the two theoretical isotherms plotted on the same graph. As seen from Table 2, from the value of R2, it is concluded that the Langmuir isotherm fits the experimental data better than Freundlich model.
The obtained results have also shown that the calculated adsorption capacity (Q0, 29.41 mg g-1) from Langmuir isotherm model exhibits good agreement with the experimental value (27.5 mg g–1). Applicability of Langmuir model describes the monolayer adsorption of U (VI) on the surface of TOAFMNPs.
The fact that the Langmuir isotherm fits the experimental data very well maybe due to homogenous distribution of active sites on the TOAFMNPs surface, since the Langmuir equation assumes that the surface is homogeneous.
| Table 2: Comparison of equilibrium isothermmodels. | |
| Langmuir | |
| Q0 (mg g-1) | 29.24 |
| b (L mg-1) | 0.256 |
| R2 | 0.990 |
| Freundlich | |
| Kf [(mg g-1)(L mg-1)1/n ] | 15.70 |
| n | 7.52 |
| R2 | 0.881 |
Selectivity of the adsorption of U(VI) at the presence of other metal ions
The effect of Al, Fe, and Mg and V ions on uranium adsorption on TOAFMNPs in synthetic sulfuric solutions wasstudied.To investigate the selective separation and adsorption of U(VI) ion from its binary synthetic solutions with diverse metal ions, an aqueous solution (50 ml) containing 10 mg g-1 U(VI) and proportional amounts of other ions according to real leach liquor condition was taken and the recommended procedure was followed.The adsorption capacity obtained from binary mixtures is presented in table 3 and Fig.10. The data showed that the TOAFMNPs were effective in selective adsorption of U(VI) from sulfuric solutions containing other metal ions.
| Table 3: Effect of maximum levels of interfering ions on the adsorption of U (VI) [60 mg of TOAFMNPs in 50ml of aqueous solutions containing 10 mg g-1 of U(VI) at pH 2 and different amount of each interfering ion at optimal experimental condition]. | |||||
|
Ions |
Source | Cons. (mg g-1) | Ions/U | Adsorptionof ions (%) | Adsorptionof U(VI) |
|
Fe2+ |
FeSO4.7H2O | 200 | 20 | 25.08 | 71.64 |
|
Al3+ |
Al(NO3)3.9H2O | 30 | 3 | 23.66 | 73.45 |
|
Mg2+ |
MgO4S.7H2O | 300 | 10 | 22.83 | 74.37 |
|
V(V) |
V2O5 | 20 | 2 | 13.75 | 76.95 |
To assess the applicability of the method to real solutions with all of diverse metal ions, it was applied to the adsorption and separation of U (VI) ions from 50 ml of synthetic sulfuric solution at pH 1 to 3.
It can see from the results according to Fig.11 that Al(III), Mg(II), Fe(III) and V(VI) are not significant found to be extracted with TOAFMNPs as adsorbent at pH <3. Thus by employing this adsorbent, U (VI) can be selectively recovered from sulfuric solution, which contains usually the above metal ions.
![Fig. 10: Effect of maximum levels of interfering ions on the adsorption of U (VI) [30, 60 mg of TOAFMNPs in 50ml of aqueous solutions containing 10 mg/l of U(VI) at pH 2 and different amount of each interfering ion at optimal experimental condition].](http://www.orientjchem.org/wp-content/uploads/2015/11/Spei_No1_Opti_Nema_Fig10-150x150.jpg) |
Figure 10: Effect of maximum levels of interfering ions on the adsorption of U (VI) [30, 60 mg of TOAFMNPs in 50ml of aqueous solutions containing 10 mg/l of U(VI) at pH 2 and different amount of each interfering ion at optimal experimental condition]. Click here to View figure |
![Fig. 11:Effect of maximum levels of interfering ions on the adsorption of U (VI) [60 mg of TOAFMNPs in 50ml of aqueous solutions containing 10 mg/l of U(VI) at pH 1,2,3 and different amount of each interfering ion at optimal experimental condition].](http://www.orientjchem.org/wp-content/uploads/2015/11/Spei_No1_Opti_Nema_Fig11-150x150.jpg) |
Figure 11: Effect of maximum levels of interfering ions on the adsorption of U (VI) [60 mg of TOAFMNPs in 50ml of aqueous solutions containing 10 mg/l of U(VI) at pH 1,2,3 and different amount of each interfering ion at optimal experimental condition]. Click here to View figure |
According to the adsorption tests, at pH 1, ions competition with together to adsorb on adsorption sites remarkably reduces the Fe and V adsorption on TOAFMNPs. When the pH increases up to 3 the Fe and V adsorption becomes increased. Al and Mg have a negligible effect on U (VI) adsorption at pH 1, however even at as high pH adsorption of Al and Mg was decreased.
Results showed that the metal ions not significant diminish the uranium adsorption on adsorbent by adsorbing themselves and competing with uranium.
When the metals are present in the solution all together with uranium, they also prevent adsorption of themselves as well as uranium. Thus, when Al, V, Fe, Mg and U are present in solution simultaneously, the adsorption of all these metals decreases.
Many other ions are dissolved with uranium from ores. Thus, after acidic leaching of ores, there are many other metal impurities in the leach solution, competing with uranium to be adsorbing on adsorbent.
Desorption of uranium and reuse of adsorbents
Desorption of U (VI) from the adsorbent was studied using different eluents (HCl, H2SO4 and NaCO3).
The adsorbent were first used in optimal adsorption experiments. 50 ml of synthetic solution was stirred with 60 mg adsorbent for 20 min. The used adsorbent was separated from solution by magnetic field and washed thoroughly with de-ionized water. Subsequently, 50 ml of the eluent was added and the mixture was stirred for 10 min. Desorption time was studied and it was found that 10 min is sufficient for metal desorption. The amount of desorbed metal ion was determined using ICP.
To evaluate the reusability of the adsorbent, three order consecutive adsorption and desorption cycles were performed. The adsorbent was washed with de-ionized water before use in subsequent adsorption and desorption cycles.
HCl, NaOH, Na2SO4, Na2CO3, HNO3, CH3COOH, H3PO4 and H2SO4 showed a satisfactory performance for desorption process of U (VI).
Desorption was tested using certain desorption agents such as sulfuric acid, hydrochloric acid and sodium carbonate. The agents’ molarities ranged from 0.2 to 2M. The following factors were studies to define the optimum conditions for desorption of U (VI) process.
When sodium carbonate was used a precipitate took place. This must probably due to the precipitation of U (VI) hydroxide at higher pH. But the precipitate was not formed at very dilute solution (0.5M) Na2CO3.
Table 4 and Fig.12 shows the effect of desorption agent concentration on the desorption efficiency of uranium.
According to results, 0.5M H2SO4 acid is the best desorption agents for uranium, (75.4%).
| Table 4: Effect of different stripping agent on desorption of uranium. | |
|
Different stripping agents and concentration |
Desorption of U(VI) % |
| Sulfuric acid (H2SO4). 0.5M |
75.4 |
| Hydrochloric Acid (HCl). 1M |
68.6 |
| Sodium Carbonate (NaCO3). 0.5 M |
57.3 |
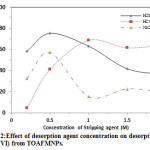 |
Figure 12: Effect of desorption agent concentration on desorption of U (VI) from TOAFMNPs. Click here to View figure |
Adsorption of Uranium from Real Sulfuric Media
Uranyl sulfate leach liquor obtained by uranium leaching of a technological sample was subjected to uranium adsorption using the MACS technique.
A uranium samplewith high iron and pyrite content was prepared from Saghand uranium mine (2th Anomaly) of Yazd provincein south of Iran. The constitute elements of ore samples obtained by X-Ray Fluorescence (XRF) spectrometry. The chemical compositions of ore sample are shown in Table 5.
According to the X-Ray Diffraction (XRD) analysis of the ore, main minerals are magnetite, pyrite, and quartz, plus uraninite as the main uranium-bearing mineral in the ore.
|
Table 5: Chemical composition of the ore sample. |
|||||
| U(%) | Th(ppm) | Fe2O3 (%) | Al2O3 (%) | MgO(%) | V2O5 (%) |
| 0.0476 | 0.0040 | 46.2 | 1.7 | 11.1 | 0.58 |
| SiO2 (%) | P2O5 (%) | Na2O (%) | K2O (%) | CaO (%) | S (%) |
| 15.4 | 0.26 | 0.16 | 0.35 | 1.3 | 2.74 |
To carried out of leaching process, Add 100 g of ore sample into 250 ml beaker. According to test conditions the oxidant, water and sulfuric acid is added. Then the beaker is put in a water bath, the slurry is agitated for a certain time as required. The pregnant liquor is separated from the slurry with vacuum filter. The filtrate collected for analysis and filter cake is washed with acidified water (pH 1.5) for three times. The filter cake is dried (under 105 °C) and pulverized for analysis.
Optimal leaching conditions were determined to be the best process for uranium extraction and shows in Fig.13 (a, b, c, d).
The present scientific study applied to uranium leaching process of the saghand ore by using sulfuric acid.
To prepare leach liquor for adsorption study, the experiment was carried out according optimal condition listed in table 6:
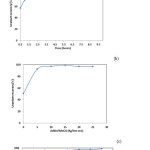 |
Figure 13: Effect of added amount of sulfuric acid (a), Time of leaching process (b), added amount of MnO2 (c) and liquid/solid ratio on recovery of uranium (d). Click here to View figure |
| Table 6: The result of leaching process of second anomaly of Saghand ore. | |||||||||
|
Test conditions |
Test result |
||||||||
| Weight of S2A ore | 0.1 Kg | Uranium recovery | 98.4% | ||||||
| Particle size | -0.5 mm | Uranium content in residue | 0.0006% | ||||||
| U grade of Ore | 0.0476 % | Weight of residue | 968.75 g | ||||||
| Acid added (H2SO4) | 100 kg/t ore | Uranium content in leach liquor 0.465 g/l | |||||||
| Oxidant added | 15 kg/t ore | No. of washing water | Volume (ml) | Uranium content | |||||
| Leaching time | 6 hours | 1st time | 1005 | 0.116 | |||||
| Leaching temperature | 50 °C | 2th time | 1010 | <0.02 | |||||
| Liquid/solid | 1/1 | 3th time | 1007 | <0.02 | |||||
|
Compositions in leached liquor |
|||||||||
| pH | 1.0 | Redox potential | 460 | mv | |||||
| H+ | 0.2 | N | U | 0.465 | g/l | ||||
| Ca | 1.0 | g/l | Mg | 7.7 | g/l | ||||
| Al | 1.04 | g/l | Fe | 6.25 | g/l | ||||
| Si | 0.273 | g/l | P | 1.36 | g/l | ||||
| SO4-2 | 45.84 | g/l | V | 1.67 | g/l | ||||
To adsorption study, the prepared 2 ml of leach liquor was contacted with 30 and 60 mg of TOAFMNPS as adsorbent in a beaker and added de-ionized water to reach 50 ml at room temperature, stir for 20 minutes. Two sets of experiments were applied for uranium adsorption at optimal conditions that these obtained from synthetic solution study.
The results in Table 7 demonstrated that a pH value of 2 with 60 mg of TOAFMNPs achieved 80.8% of uranium adsorption with clear separation from other associated elements, iron, vanadium, aluminum and magnesium.
| Table 7:Adsorption of metal ions from second anomaly of saghand leach liquor using TOAFMNPs as novel adsorbent | ||
|
Metal ions |
Adsorption (%) of U(VI) Using 30 mg TOAFMNPs |
Adsorption (%) of U(VI) Using 60 mg TOAFMNPs |
|
U |
59.7 |
80.8 |
|
Fe |
9.4 |
10.9 |
|
Al |
23.6 |
16.4 |
|
Mg |
28.1 |
17.3 |
|
V |
11.8 |
17.9 |
Uranium was effectively adsorbed from sulfate leach liquor by TOAFMNPs as a novel adsorbent.
Conclusion
A novel adsorbent based on magnetite nanoparticles was synthesized and functionalized with TOA. The application of the functionalized magnetite nanoparticles as an adsorbent in the adsorption of U (VI) from synthetic sulfuric solutions was investigated. The optimum conditions were achieved and it was applied for real sulfuric solutions.
The leach solution from 2th anomaly of Sag hand ore mine was prepared and the adsorption of uranium was carried out by TOAFMNPs.
Uranium ore processing was successfully applied with 80.8% uranium recovery with negligible other elements (iron, vanadium, aluminum and magnesium) interferes.
References
- Kaminski, M.D.; Nunez. L.; J. O. Mag. Mag. Mat. 1999,194, 31-36.
- Shaibu, B.S.; Reddy, M.L.P.; Bhattacharyya, A.;Manchanda, V.K. J.O. Mag. Mag. Mat.2006,301, 312–318.
- Petrova, T. M.; Fachikov, L.; Hristov, J. I. R. E. C. H. E.2011,3, 134-152.
- Olmes, A. S.; Akyil, S.; Eral, M. J. Radio. Nucl. Chem. 2004, 260, 119–125.
- Nibou, D.; Khemaissia, S.; Amokrane, S.; Barkat, M.; Chegrouche, S.; Mellah, Inter. Rev.O. Chem. Eng. 2012,4, 3.
- Leslie-Pelecky, D. L.; Rieke, R. D. J. Chem. Mater.1996, 8, 1770–1783.
- Devi, M.; Fingerman, M. B. Environ. Contam. Tox. 1995,55, 746–750.
- Zhang, F. S.; Nriagu, J. O.; Itoh. H. J. Water. Res. 2005,39, 389–395.
- Uzun, L.; Kara, A.; Tüzmen, N.; Karabakan, A.; Besirli, N.; Denizli, A. J. Appl. Polym. Sci. 2006, 102, 4276–4283.
- Chen, C. Y.; Lin, M. S.; Hsu, K. R. J. Hazard. Mater. 2008, 152, 986–993.
- Rivas, B. L.; Pooley, S. A.; Maturana, H.A.; Villegas, S. J. Macromol. Chem. Phys. 2001, 202, 443–447.
- Zhou, D.; Zhang, L.; Zhou, J.; Guo. S. J. Water. Res. 2004, 38, 2643–2650.
- Nunez, L.; Kaminski, M.; Bradley, C.; Buchholz, B.A.; Landsberger, S. B.; Aase, S.; Tuazon, H.E. Argonne National Laboratory, Chemical Technology Division. 1995, ANL-95/1.
- Nunez, L.; Kaminski, M.D. J. O. Mag. Mag. Mat. 1999,194, 102-107.
- Buchholz, B. A.; Tuaazon, H. E.; Kaminski, M. D.; Aase, S. B.; Nunez, L.; Vandegrift. G. F. J. Sep and Pur. Tech. 1997, 11. 21l-219.
- Nunez, L.; Buchholz, B.A.; Ziemer, M.; DyrKacz, G.; Kaminski, M.; Vandergrift, G.F.; Atkins. K. Argonne National Laboratory, Chemical Technology Division.1994, ANL-94/47.
- Nunez, L.; Kaminski, M.; Bradley, C.; Buchholz, B. A.; Landsberger, S.; Aase, S. B.;Tuazon, H. E.; Vandergrift, G.F. Argonne National Laboratory, Chemical Technology Division.1995, ANL-95/26.
- Sadeghi, S.; Azhdari, H.; Arabi, H.; Moghadam. A. Z. J. hazardeous. materials. 2012,216, 208-216.
- Leal, R.; Yamaura. M. J.O. Nuclear. Ener. Scien. Tech. 2011,6, 1-7.
- Nigond, L.; Musikas, C.; Cuillerdier, C.; Solvent Extr. Ion Exch. 1994, 12, 297.
- Nair, G. M.; Prabhu, P. R.; Mahajan, G. R.; J. Radioanal. Nucl. Chem. 1994, 182, 393.
- Nair, G. M.; Mahajan, G. R.; Prabhu, P. R.; J. Radioanal. Nucl. Chem. 1995, 191, 323.
- Nakamura, T.; Miyake, C.; Solvent Extr. Ion Exch. 1995, 13, 253.
- Preston, J. S.; Du Preez, A. C.; Solvent Extr. Ion Exch. 1995, 13, 391.
- Ruikar, P. B.; Nagar, M. S.; Subramanian, M. S.; Gupta, K. K.; Varadarajan, N.; Singh, R. K.; J. Radioanal. Nucl. Chem. 1995, 196, 171.
- Moore, F. L.; Anal. Chem. 1958, 30, 908.
- Južnič, K.; Fedina, S.; Mikrochim. Acta1974, 62, 39.
- Goldenberg, J. F.; Abbruzzese, C.; Int. J. Miner. Process. 1983, 10, 241.
- Lyle, S. J.; Tamizi, M. A.; Hydrometallurgy.1983, 11, 1.
- Ito, K.; J. Radioanal. Nucl. Chem. 1993, 171, 371.
- Behera, P.; Mishra, R.; Chakravortty, V.; J. Radioanal. Nucl. Chem. 1993, 173, 161.
- Kumar, J. R.; Lee, J. Y.; Kim, J. S.; Yoon, H. S.; J. Radioanal. Nucl. Chem. 2010, 285, 301.
- Yakubu, N. A.; Dudeney, A. W. L.; Hydrometallurgy. 1987, 18, 93.
- Collet, S.; Chagnes, A.; Courtaud, B.; Thiry, J.; Cote, G.; J. Chem. Techol. Biotechnol. 2009, 84, 1331.
- Kumar, J. R.; Kim, J. S.; Lee, J. Y.; Yoon, H. S.; Sep. Purif. Rev. 2011, 40, 77.
- Mansouri, N.; Saberyan, K.; Noaparast, M; J.O.Advaces. Chem. 2014,10, 2403-2414.
- Klug, H.P. and Alexander, L.E.,X-ray Diffraction Procedures for Polycrystalline and Amorphous Materials, John Wiley and Sons. New York, p. 491. (1962).
- Das, D.; Sureshkumar, M. K.; Koley, S.; Mithal, N.; Pillai, C.G. S. J. chem. Thermodyn. 2010, 41, 829-835.
- Faraji, M.; Yamini, Y.; Rezaee, M. J. Talanta. 2010, 81, 831–836.

This work is licensed under a Creative Commons Attribution 4.0 International License.









