One-pot synthesis of indeno-fused spirooxindoles catalyzed by SBA-Oxime-Zn
Sayed Hosein Ghorbani* and Mohammad Mahmoodi Hashemi
Department of chemistry, Science and Research Branch, Islamic Azad University, Tehran, Iran
Corresponding Author Email: ghorbani3551@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/31.Special-Issue1.22
Article Received on :
Article Accepted on :
Article Published : 06 Nov 2015
An extremely efficient catalytic protocol for the synthesis of spirooxindole derivatives via a one-pot three component approach in the presence of SBA-Oxime-Zn, as a heterogeneous catalyst using water as a green solvent is reported. The catalyst exhibited excellent catalytic activity, and the proposed methodology is capable of providing the desired products in good yield and short reaction time. After reaction course, the catalyst can be recycled and reused without any apparent loss of activity which makes this process cost effective and hence eco-friendly.
KEYWORDS:extremely efficient; One-pot; spirooxindoles
Download this article as:| Copy the following to cite this article: Ghorbani S. H, Hashemi M. M. One-pot synthesis of indeno-fused spirooxindoles catalyzed by SBA-Oxime-Zn. Orient J Chem 2015;31(Special Issue1). |
| Copy the following to cite this URL: Ghorbani S. H, Hashemi M. M. One-pot synthesis of indeno-fused spirooxindoles catalyzed by SBA-Oxime-Zn. Orient J Chem 2015;31(Special Issue1). Available from: http://www.orientjchem.org/?p=12258 |
Introduction
One of the essential features in (green) chemistry is closely related to the number of steps in organic synthesis as well as atom economy. Hence, multi-component reactions (MCRs) are becoming a growingly important class of reactions by virtue of their convergence, productivity, facile execution and high yield [[i]]. Applications of MCRs in drug discovery, material sciences, natural product synthesis, and ligand and biological probe preparations further prove the power of this reaction [[ii],[iii]]. Combining the aspects of MCRs with heterogeneity and catalysis would reinforce the “greenness” of the reactions. Furthermore, the demand for environmentally benign procedures promoted the use of heterogeneous catalysts to achieve. Heterogeneous catalysts are always superior to their homogeneous counterparts in terms of many aspects such as effective catalyst handling, easy isolation of products, reusability, environmental compatibility and high selectivity. Therefore, introducing clean processes and utilizing eco-friendly and green catalysts, which can be simply recycled at the end of reactions, have been under permanent attention.
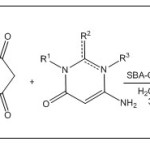 |
Scheme Click here to View scheme |
One of the key principles of green chemistry [4] is to eliminate the use of hazardous solvents and their replacement with environmentally benign solvents. Water emerged as a unique alternative solvent for several organic reactions owing to many of its potential characteristics such as safety, economy and environmental concerns [5]. In some cases the strong hydrogen bonding ability of water leads to higher reactivity and selectivity in comparison to other conventional organic solvents [6],[7].
Spirooxindole fragments are found in a large number of natural products and medicinal agents [8]and a great deal of attention has been paid to them due to their structural relevance with bioactive molecules [9].Since the creation of a quaternary center is considered a difficult task in synthetic organic chemistry, the spirooxindole synthesis becomes more challenging [10]. Among the “drug-like” small molecules, due to exclusivity of biological modes of action and the therapeutic promise of spirooxindoles, the development of innovative approaches to their synthesis is rapidly growing. [11].
In this work an attempt has been made to synthesize spirooxindole derivatives in the presence of SBA-Oxime-Zn as a heterogeneous catalyst in water.
Results and Discussion
Initially, the SBA-15 was allowed to react with excess of thionyl chloride under reflux conditions, to afford the corresponding surface chlorinated SBA-Cl [12]. Picolinaldehyde oxime was used for organic modification of SBA-15 surface. Finally, silica immobilized zinc complex was prepared by stirring the appropriate silica support with solution of ZnCl2 in toluene at room temperature. The tentative structure of the catalyst is shown in Scheme 1.
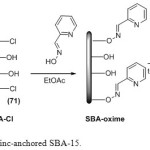 |
Scheme 1a: Synthesis of zinc-anchored SBA-15. Click here to View scheme |
The catalyst structure was characterized by FT-IR spectroscopy, thermo gravimetric analysis (TGA), and atomic absorption spectroscopy. The FT-IR spectra of SBA-15, SBA-oxime and SBA-Oxime-Zn are shown in Fig. 1. In all spectrums, the typical Si–O–Si bandsaround 459, 811 and 1023cm-1 associated with the formation of a condensed silica network. The FT-IR of SBA-oxime shows peak due to C=N around 1636 cm−1, which on complexation with zinc shifts to lower value (1619 cm−1) [3]. The lowering in frequencies of the C=N peak is indicative of the formation of metal–ligand bond.
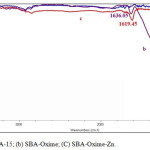 |
Figure 1: IR spectra of (a) SBA-15; (b) SBA-Oxime; (C) SBA-Oxime-Zn. Click here to View figure |
The thermal stability of the prepared sample was investigated by a TGA method. The thermogram obtained for the SBA-oxime-Zn is presented in Fig. 2. The TGA profile was characterized by weight loss till 220° C and the catalyst showed good thermal stability. The zinc content of the catalysts was estimated to be 0.17 mmol g-1 by atomic adsorption spectroscopy.
After characterization of the catalyst, its catalytic efficiency has been systematically studied in the rapid and green preparation of spirocyclic oxinoldes in water through a multi-component reaction. For this purpose, in a prototype experiment, we focused on a three-component reaction of isatin 1a, 1,3-indandione 2 and 4-aminouracil 3a as a model reaction (Table 1). To establish our optimum conditions, different solvents, temperatures and catalyst amounts were investigated. As can be seen from Table 1, the best result was obtained with a 0.1 g catalyst in refluxing water.
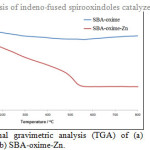 |
Figure 2: Thermal gravimetric analysis (TGA) of (a) SBA-oxime and (b) SBA-oxime-Zn. Click here to View figure |
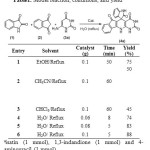 |
Table 1: Model reaction, conditions, and yield a Click here to View table |
aisatin (1 mmol), 1,3-indandione (1 mmol) and 4-aminouracil (1 mmol).
With the optimized reaction conditions in hand, a series of indeno-fused spirooxindoles were prepared via three-component reaction of isatins, 1-3-indandion and 4-aminouraciles and variety of the desired products were obtained in good yields for 30 minutes (Table 2).
The reusability of the catalyst in the reaction of isatin, 1,3-indandione and 4-aminouracil in refluxing water was evaluated. In this procedure, upon completion of the reaction, the mixture was cooled to room temperature and the crude product was filtered off and hot ethanol was added. Then, the catalyst was filtered and washed with ethanol, dried at 60 oC for 4 h and reused. The catalyst can be reused for up to three cycles without significant loss in its activity (Fig. 3).
 |
Table 2: Catalytic activity of SBA-oxime-Zn in synthesis of spirooxindoles 4. Click here to View table |
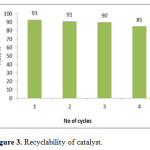 |
Figure 3: Recyclability of catalyst. Click here to View figure |
Conclusion
To summarize, we have successfully immobilized the Zinc complex on SBA-15 matrix via covalent bond. The catalyst as shown excellent catalytic activities in condensation reaction of isatin, 1,3-indandion and enamine compounds. The promising points of this method are clean reaction protocol, short reaction times, simplicity in operation, high catalytic activity and good recyclability of the catalyst. Above all, because some of the existing catalytic methods suffer from lack of stability and recoverability in aqueous medium, the improvement of a catalytic system that is not only stable toward water but also easily recyclable is highly desirable.
Materials and Methods
The chemicals were purchased from Fluka AG, Merck, and Aldrich companies and used without further purification. FTIR spectra were obtained using a BOMEM MB-series FT-IR apparatus using KBr pellets. 1H NMR spectra were recorded with a 300 MHz Bruker DRX Avance spectrometer. The copper content of the sample was estimated on a Varian Shimadzu AA-680 atomic absorption spectrometer. Thermo gravimetric analyses (TGA) were recorded on a TG 209 F1 Netzsch. Melting points were recorded on a Thermo Scientific 9100 apparatus in open capillary tubes. SBA-15 was synthesised according previously reported method [15].
Synthesis of SBA-Cl
To an oven-dried (120 °C) SBA-15 (10 g) in around bottomed flask (250 mL) equipped with a condenser and a drying tube, was added thionyl chloride (40 mL) and refluxed for 48 h. The excess of thionyl chloride was distilled off. The resulting white-greyish powder was flame dried and stored in a tightly capped bottle.
Synthesis of oxime-grafted SBA-15 (SBA-oxime)
Organic modification of SBA-Cl has been performed by stirring 0.5 g of SBA-Cl with picolinaldehyde oxime (1.5 mmol, 0.18 g) in dry ethyl acetate (10 mL) at room temperature for 48 h. The SBA-Oxime as cream solid was filtered and washed thoroughly with copious amount of ethyl acetate, chloroform and acetone. Then, the SBA-oxime was dried at 60 oC for 5 h.
Preparation of SBA-oxime supported copper catalyst (SBA-oxime-Zn)
SBA-oxime supported zinc catalyst was prepared by stirring the SBA-oxime support (0.5 g) with a solution of the ZnCl2 (0.5mmol) in dry toluene (10 mL) at room temperature for 24 h. The cream solid was filtered, and washed with acetone and methanol. The SBA-oxime-Zn was dried at 50 oC for 5 h.
General procedure for the preparation of spirooxindoles
A mixture of isatin 1 (1 mmol), 1,3-indandion 2 (1 mmol), aminouracil 3 (1 mmol) and SBA-oxime-Zn (0.1 g) was heated in refluxing water (5 mL) for appropriate time (Table 1). The reaction was monitored by TLC (n-hexane-ethyl acetate 1:1), and after completion of the reaction, the reaction mixture was filtered for separation of solid product and catalyst from water. The remaining solid was washed with ethanol (2×10 mL) for separation of the product from the catalyst. Finally, the product was purified by recrystallization in hot EtOH. The desired pure products were characterized by comparison of their melting point data with literature.
All the products are known compounds and their melting points are compared with reported values [14]. Some selected samples were also characterized by IR and NMR spectroscopic data.
Selected spectral data
1H-spiro[indeno[1,2-b]pyrido[2,3-d]pyrimidine-5,3′-indoline]-2,2′,4′,6′(3′H,10′H)-tetraone(4a
Orange powder (89%); mp 293-295°C.IR (KBr) (vmax, cm-1): 3358, 3163, 1736, 1696, 1657. 1H NMR (300 MHz, DMSO-d6): δH (ppm) 6.82-7.56 (8H, m, H-Ar), 10.46 (1H, s, NH), 10.58 (2H, m, 2NH), 10.90 (1H, s, NH).
5′-bromo-1H-spiro[indeno[1,2-b]pyrido[2,3-d]pyrimidine-5,3′-indoline]-2, 2′,4,6(3′H,10′H)-tetraone (4b):
yellow powder (yield 80%); mp >300 °C. IR (KBr) (vmax, cm-1): 3390, 3217, 1751, 1720, 1649, 1602. 1H NMR (300 MHz, DMSO-d6): 6.71-7.61 (7H, m, H-Ar), 10.52 (2H, s, 2NH), 10.56 (1H, s, NH), 10.96 (1H, s, NH).
1′-methyl-1H-spiro[indeno[1,2-b]pyrido[2,3-d]pyrimidine-5,3′-indoline]-2,2′,4,6 (3′H,10′H)-tetraone (4c):
light orange powder (yield 76%); mp >300 °C. IR (KBr) (vmax, cm-1): 3358, 3232, 1737, 1699, 1647, 1603. 1H NMR (300 MHz, DMSO-d6): δH(ppm) 3.15 (3H, s, CH3), 6.88-7.50 (8H, m, H-Ar), 10.70 (1H, s, NH), 10.90 (1H, s, NH).
1-methyl-1H-spiro[indeno[1,2-b]pyrido[2,3-d]pyrimidine-5,3′-indoline]-2,2′,4,6 (3′H,10′H)-tetraone (4d):
Yellow powder (yield 89%); mp >300 °C. IR (KBr) (vmax, cm-1): 3332, 33061, 1763, 1696, 1632. 1H NMR (300 MHz, DMSO-d6): δH(ppm) 3.52 (3H, s, CH3), 6.99-7.63 (8H, m, H-Ar), 10.82(1H, s, NH), 10.95 (1H, s, NH), 11.59 (1H, s, NH).C NMR (75 MHz, DMSO-d
1-methyl-5′-nitro-1H-spiro[indeno[1,2-b]pyrido[2,3-d]pyrimidine-5,3′-indoline]-2, 2′,4,6(3’H,10’H)-tetraone (4e)
Orange powder (yield 93%); mp >300 ˚C. IR (KBr) (vmax, cm-1): 3351, 3238, 1766, 1743, 1661, 1615. 1H NMR (300 MHz, DMSO-d6): δH(ppm): 3.55 (3H, s, CH3),6.99-8.09 (7H, m, H-Ar), 11.00 (1H, s, NH), 11.47 (1H, s, NH), 11.84 (1H, s, NH).
1,1′,3-trimethyl-1H-spiro[indeno[1,2-b]pyrido[2,3-d]pyrimidine-5,3′-indoline]- 2,2′,4,6 (3′H,10′H)-tetraone (4i)
Orange Powder (yield 79%); mp >300 °C. IR (KBr) (vmax, cm-1): 3332, 3089, 1756, 1732, 1669, 1609. 1H NMR (300 MHz, DMSO-d6): δH(ppm) 3.00 (3H, s, CH3), 3.170 (3H, s, CH3), 3.91(3H, s, CH3), 6.87-7.89 (8H, m, H-Ar), 11.73 (1H, s, NH).
2-amino-3H-spiro[indeno[1,2-b]pyrido[2,3-d]pyrimidine-5,3′-indoline]-2,2′,4,6(3′H,10′H)-tetraone (4k):
Light orange powder (95%); mp 266-268 °C.IR (KBr) (vmax, cm-1): 3331, 3036, 1725, 1688, 1635. 1H NMR (300 MHz, DMSO-d6): δH (ppm) 6.59(2H, s, NH2), 6.80 -7.90 (8H, m, H-Ar), 10.30 (1H, s, NH), 10.63 (1H, s, NH), 11.00 (1H, s, NH).
References
- (a) Zhu, J.; Bienaymé, H. Multicomponent Reactions, Wiley-VCH, Weinheim, 2005; (b) Dömling, A.; Ugi, I. Angew. Chem. Int. Ed. 2000, 39, 3168.
- Groenendaal, B.; Vugts, D. J.; Schmitz R. F. J. Org. Chem. 2008, 73, 719.
- Groenendaal, B.; Ruijter, E.; Orru, R. V. A. Chem. Commun. 2008, 5474.
- (a) Anastas, P. T.; Warner, J. C. in Green Chemistry Theory and Practice, Oxford University Press, Oxford, 1998;(b) Matlack, A. S. Introduction to Green Chemistry, Marcel Dekker, New York, 2001; (c) Handbook of Green Chemistry and Technology, Clark, J. H.; Macquarrie, D. J. Eds., Blackwell Publishing, Abingdon, 2002; (d) Lancaster, M. Green Chemistry: An Introductory Text, RSC Publishing, Cambridge, 2010.
- (a) Nelson, W. M. Green Solvents for Chemistry: Perspectives and Practice, Oxford University Press, New York, 2003. (b) Clark J. H.; Taverner, S. J. Org. Process Res. Dev.2007, 11, 149; (c) Kerton, F. M. Alternative Solvents for Green Chemistry, RSC Publishing, Cambridge, 2009.
- Li, C. J. Chem. Rev., 2005, 105, 3095.
- Bazi, F.; Badaoui, H.; Tamani, S.; Sokori, S.; Solhy, A.; Macquarrie D. J.; Sebti, S. Appl. Catal., A 2006, 211, 301.
- (a) Lin, H.; Danishefsky, S. J. Angew. Chem., Int. Ed.2003, 42, 36; (b) Marti, C.; Carreira, E. M. Eur. J. Org. Chem. 2003, 2209; (c) Lo, M. M. C.; Neumann, C. S.; Nagayama, S.; Perlstein, E.O.; Schreiber, S. L. J. Am. Chem. Soc.2004, 126, 16077; (d) Chen, C.; Li, X. D. ;Neumann, C. S.; Lo, M. M. C.; Schreiber, S. L. Angew. Chem., Int. Ed. 2005, 44, 2249; (e) Ding, K.; Lu, Y.; Nikolovska-Coleska, Z.; Qiu, S.; Ding, Y.; Gao, W.; Stuckey, J.; Krajewski, K.; Roller, P. P.; Tomita, Y.; Parrish, D. A.; Deschamps, J. R.; Wang, S. J. Am. Chem. Soc. 2005, 127, 10130; (f) Yeung, B. K. S.; Zou, B.; Rottmann, M.; Lakshminarayana, S. B.; Ang, S. H.; Leong, S. Y.; Tan, J.; Wong, J.; Keller-Maerki, S.; Fischli, C.; Goh, A.; Schmitt, E. K.; Krastel, P.; Francotte, E.; Kuhen, K.; Plouffe, D.; Henson, K.; Wagner, T.; Winzeler, E. A.; Petersen, F.; Brun, R.; Dartois, V.; Diagana, T. T.; Keller, T. H. J. Med. Chem.2010,53, 5155.
- (a) Han, C.; Zhang, T.; Zhang, A.; Wang, D.; Shi, W.; Tao, J. Synthesis 2014, 1389; (b) Matsumaru, T.; McCallum, M. E.; Enquist Jr., J. A.; Smith, G. M.; Kong, K.; Wood, J. L. Tetrahedron 2014, 70, 4089; (c) Santos, M. M. M. Tetrahedron2014, 70, 9735; (d) Zhao, Y.; Liu, L.; Sun, W.; Lu, J.; McEachern, D.; Li, X.; Yu, S.; Bernard, D.; Ochsenbein, P.; Ferey, V.; Carry, J.-C.; Deschamps, J. R.; Sun, D.; Wang, S. J. Am. Chem. Soc.2013, 135, 7223.
- Hudlicky, T.; Reed, J. W. The Way of Synthesis; Wiley-VCH:Weinheim. 2007, p. 98.
- For some selected syntheses of spirocarbocyclic oxindoles, see: (a) Mercado-Marin, E. V.; Garcia-Reynaga, P.; Romminger, S.; Pimenta, E. F.; Romney, D. K.; Lodewyk, M. W.; Williams, D. E.; Andersen, R. J.; Miller, S. J.; Tantillo, D. J.; Berlinck, R. G. S.; Sarpong, R. Nature 2014, 509, 318; (b) Ding, L. -Z.; Zhong, T.-S.; Wu, H.; Wang, Y.-M. Eur. J. Org. Chem. 2014, 5139; (c) Zhou, Q.-Q.; Yuan, X.; Xiao, Y.-C.; Dong, L.; Chen, Y.-C. Tetrahedron 2013, 69, 10369; (d) Jiang, K.; Jia, Z.-J.; Chen, S.; Wu, L.; Chen, Y.-C. Chem. Eur. J.2010, 16, 2852; (e) Wang, Y.; Liu, L.; Zhang, T.; Zhong, N. -J.; Wang, D.; Chen, Y. -J. J. Org. Chem. 2012, 77, 4143; (f) Sun, W.; Zhu, G.; Wu, C.; Hong, L.; Wang, R.; Chem. Eur. J. 2012, 18, 13959; (g) Lingam, K. A. P.; Shanmugam, P.; Selvakumar, K. Synlett 2012, 23, 27; (h) Lingam, K. A. P.; Mandal, A. B.; Shanmugam, P. Tetrahedron Lett. 2011, 52, 3610; (i) Richmond, E.; Duguet, N.; Slawin, A. M. Z.; Lebl, T.; Smith, A. D. Org. Lett. 2012, 14, 2762; j) Zhong, F.; Han, X.; Wang, Y.; Lu, Y. Angew. Chem. 2011, 123, 7983; Angew. Chem. Int. Ed. 2011, 50, 7837; (k) Bencivenni, G.; Wu, L.-Y.; Mazzanti, A.; Giannichi, B.; Pesciaioli, F.; Song, M.-P.; Bartoli, G.; Melchiorre, P. Angew. Chem. 2009, 121, 7336; Angew. Chem. Int. Ed. 2009, 48, 7200; (l) Shi, X. -M.; Dong, W. -P.; Zhu, L. -P.; Jiang, X. -X.; Wang, R. Adv. Synth. Catal. 2013, 355, 3119.
- Karimi, B.; Behzadnia, H. Synlett; 2010, 2010, 2019.
- Paul, S.; Clark, J. H. J. Mol. Catal. A. Chem.; 2004, 215, 107.
- Imani Shakibaei, G.; Feiz, A.; Khavasi, H. R.; Abolhasani Soorki, A.; Bazgir,A.ACS Comb. Sci. 2011, 13, 96.
- Das, D.; Tsai, C.-M.; Cheng, S. Chem. Commun.; 1999, 473.

This work is licensed under a Creative Commons Attribution 4.0 International License.









