Conditions for the improvement of protective properties of the oxide coatings formed on the steel in the liquid lead-bismuth and lead
Konstantin Dmitrievich Ivanov, Olga Vladimirovna Lavrova and Said-Ali Sabirovich Niyazov
Joint Stock Company, State Scientific Centre of the Russian Federation – Institute for Physics and Power Engineering named after A.I. Leypunsky (JSC "SSC RF-IPPE") Russia, 249033 Kaluga region, Obninsk, Bondarenko, 1
DOI : http://dx.doi.org/10.13005/ojc/31.Special-Issue1.08
Article Received on :
Article Accepted on :
Article Published : 07 Nov 2015
No Abstract
KEYWORDS:Conditions; improvement; protective
Download this article as:| Copy the following to cite this article: Ivanov K. O, Lavrova O. V, Niyazov S. S. Conditions for the improvement of protective properties of the oxide coatings formed on the steel in the liquid lead-bismuth and lead. Orient J Chem 2015;31(Special Issue1). |
| Copy the following to cite this URL: Ivanov K. O, Lavrova O. V, Niyazov S. S. Conditions for the improvement of protective properties of the oxide coatings formed on the steel in the liquid lead-bismuth and lead. Orient J Chem 2015;31(Special Issue1). Available from: http://www.orientjchem.org/?p=12398 |
Introduction
Effect of spontaneous enrichment of the oxide layer with alloying additives that improve protection properties of the oxide coating, has long been known and called “corrosion induced auto-protection” [6]. In particular, it is indicative for the high temperature oxidation modes of chromium steels and chromium-nickel alloys in the air and carbon dioxide atmosphere [3].
As for steel oxidation in heavy liquid metal coolants (HLMC), auto-protection effect is occasionally revealed on the steels with high content of chromium and silicon.
For instance, it was this effect that, in the opinion of the authors of [16], caused significant decrease in the corrosion rate of the hot sections of EP 823 steel cladding (as compared to that of its low temperature sections) of the fuel element mock-up tested in the liquid lead-bismuth coolant flow during 1500 hours, min and max temperatures being, respectively, equal to 300ºС and 600ºС. However, no reasonable explanation of this process mechanism is given in this publication.
There is a lot of experimental data on the enrichment of the oxide films with silicon. As an example, in [4] presented are the results of studies on EP 823, Т- 91, 1.4970 and Optifer IV steels tested in lead-bismuth coolant at 470ºС during 3116 hours with oxygen concentration in the coolant equal tо (1-2) 10-6 wt.%. In this publication, authors claimed that the beneficial effect of silicon on corrosion resistance of steels was clearly pronounced. However, no noticeable influence of the other alloying additives was revealed, and a conclusion was made on the necessity for special studies.
Significant values of the oxide film enrichment with not only silicon, but also chromium (СCr ~ 45 wt.%) were detected by the authors of [2] for EP 823 steel tested in Pb-Bi coolant at 600ºС , this once during much longer time (~ 50000 hours).
On the other hand, it is noted in many publications, such as [9-12], that oxidation of Т- 91 and AISI 316L steels held in oxygen saturated lead-bismuth at the temperatures from 500ºС to 560ºС during 1000 hours and 2832 hours did not result in any significant enrichment of the oxide films with chromium and silicon.
In [13], given are the results of testing of SIMP steel with high content of chromium and silicon (СCr ~ 10.8 wt.%, CSi ~ 1.43 wt.%) by its exposure to Pb-Bi coolant along with the above Т-91 steel (СCr ~ 9 wt.%, CSi ~ 0.2 wt.%). Exposure time was 300, 500 and 1000 hours at 600ºС. It was revealed that the parabolic rate constant of SIMP steel oxidation turned out to be almost an order of magnitude lower than that for Т- 91 steel, while high concentrations of both chromium and silicon (СCr ~ 24 wt.%, CSi ~ 3 wt.%) were detected in the oxide film. On the other hand, chromium and silicon enrichment of the oxide films on T-91 steel was near zero.
It is appropriate at this point to make a note, which is important for better understanding of the term “enrichment”. This is about the interpretation of the results of microanalysis of the elements present in the oxide films, mostly showing higher weight content of chromium and silicon as compared to that in the steel. However, conversion from weight percent to volume percent leads to that the seeming content increase disappears, if there is no enrichment. This action was used, in particular, by the authors of [8] for the analysis of the results of Т-91 steel oxidation in lead-bismuth.
Taking into account the uncertainty of manifestation of steel auto-protection effect, as well as the absence of the reasonable explanation of the above phenomena, conditions for implementation of this effect are considered in this article.
Research and development work was carried out with state financial backing by the Ministry of Education and Science of the Russian Federation (unique identifier of the applied research is RFMEF162614X0002).
Derivation of basic analytical relationships for evaluation of the oxide films enrichment with the alloying components
We would proceed from the premise that corrosion auto-protection process under consideration is due to the natural oxidation progress in time. It is implemented owing to spontaneous enrichment of the oxide layer with the alloying components on the inner oxide layer – steel matrix interface, i.e. the increase in the volumetric concentration of the alloying element in the oxide film over that in the steel. In Figure1 presented is the simplified diagram of the oxidation process. Simplification means the absence of the intermediate area between the steel matrix and the oxide film, i.e. steel boundary coincides with the internal boundary of the oxide film, although in general it is not true.
 |
Figure 1: General diagram of typical oxidation process at any time Click here to View figure |
In Figure 1, initial steel-coolant interface position is shown by dashed line (to=0). The oxide layer being formed is located on both sides of the initial interface. At any arbitrary point of time , thickness of the oxide layer penetrating to the steel matrix is δinner, and thickness of the oxide layer penetrating outside is δouter. The total oxide layer thickness is δ = δinner + δouter.
Let us consider penetration of the oxide layer boundary into the steel matrix.
Oxygen flows from the oxide film formed earlier and metallic components flows from the steel aimed at the boundary under consideration assure formation of the oxide layer on this boundary, and its growth rate can be expressed as follows:
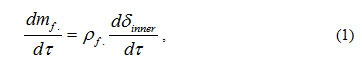
where mf. – mass of material in the oxide film being formed on the boundary under consideration per unit surface;
Pf. – density of the oxide film material;
dδinner/dt -rate of the oxide film penetration into the steel matrix.
It is evident that only those alloying components may be embedded into the oxide layer on the inner boundary, which are capable of forming oxide compounds under specific conditions, i.e. under partial oxygen pressure in the reaction zone not exceeding that above the “iron-magnetite” or “iron-wustite” equilibrium system depending on temperature. Flows of these alloying elements to their chemical reaction zone are designated by j subscript [7].
Degree of enrichment of the growing oxide layer with the components, which, along with the iron, are embedded into the oxide film, can be evaluated on the basis of mass balance of these components in the assumption of their full adoption by the oxide film. Then the flow connection equation for j-th component in the oxide layer formation area can be described as the sum of two flows: i) main flow determined by the total steel oxidation rate on the inner boundary, and ii) additional flow related to diffusion mobility of the alloying element in the steel:
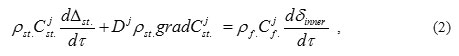
where Cj – concentrations of j-th component in the steel and in the oxide film;
Δst – steel wall thickness;
Dj(T)- coefficient of j-th component diffusion in the steel.
In order to obtain manifested physical content of the equations derived further, some nonessential assumptions can be made, namely: this component in the reaction zone is fully converted to the oxide compound, and the rate of the steel wall thickness decrease is equal to the rate of penetration of reaction zone to the matrix body dΔst/dt = dδinner/dt.
Within the framework of these assumptions, it is follows from the relationship (2) that j-th component concentration in the oxide film Cjf during its formation can be presented as follows:
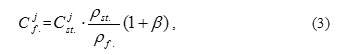
where β – coefficient, which is determined by the following relationship:
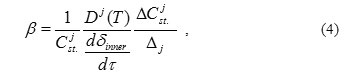
characterizes degree of the oxide film enrichment with the corresponding component. In addition to the designations used earlier, there is a new designation Δj of diffusion route of j-th component in the steel.
Thus, coefficient β is the ratio of the rate of j-th alloying component diffusion penetration to the reaction zone
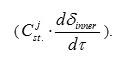
to the mass rate of stationary chromium oxidation owing to the reaction front penetration into the steel matrix
The former parameter characterizes diffusion induced capability of component transportation from the steel matrix to the reaction zone, while the latter determines time restrictions for manifesting this diffusion induced capability.
Numerical values of β parameter are ranging from zero (no enrichment) to some max value corresponding to formation of pure oxide of j-th component. In particular, in case of formation of Cr2O3 oxide (CCr = 68 wt.%, P=5.21g/cm3) on the steel containing 12% chromium, it follows from relationship (3) that
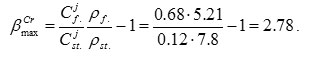
Analysis of derived relationship (3) taking into account relationship (4) shows that:
- if diffusion mobility of the alloying component, such as silicon, is high, then the oxide film enrichment with this component may occur already in the initial stage of the oxidation process, in spite of the fact that in this case relatively high steel oxidation rates are usually realized because of insignificancy of formed diffusion layer;
- on the contrary, for the alloying components with moderate diffusion mobility in the steel (such as chromium), this effect would be observed in the further oxidation stages, and, under particular conditions it may fail to manifest during observation time.
Effect of temperature factor on the enrichment process
Effect of temperature factor on the enrichment process can be evaluated by substituting corresponding numerical values to the relationship (4).
Indeed, remembering generally accepted expression for diffusion coefficient:
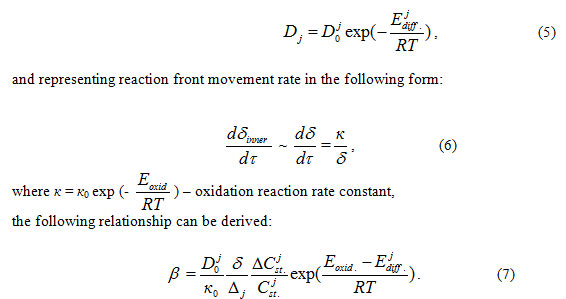
Thus, at fixed temperature value, only oxide film thickness δ=δ (t) is the variable depending on the degree of the oxidation progress.
In this case, temperature factor of the oxide layer enrichment with j-th component is characterized by the difference of energy of activation of the alloying element diffusion caused delivery to the reaction zone and that of the oxidation process itself [5].
As the steels with relatively high chromium content (8 – 18% Cr), whose corrosion resistance being primarily is caused by chromium containing oxides, have been chosen by now for the use in HLMC, let us consider the possibility of realization of auto-protection mechanism by means of this particular alloying element as applied to EP 823 steel used as cladding material for the fuel elements cooled by the lead-based heavy liquid metals. Taking into account diffusion coefficient of chromium is the main challenge, since it has not been determined for EP 823 steel.
Its numerical values can be calculated for temperatures up to ~ 6000С using data from [12] where typical steel oxidation rates are presented for this temperature range as flows of iron built into the oxide film composition.
These are experimentally determined, hypothetically for oxidation stage characterized by formation of FeCr2O4
oxide layer (СCr ~ 45 wt.%., P =4.79g/cm3, β=1.3) with iron flow of ~ 10-7 gFe/Cm2/hr on the boundary with steel matrix. Then, substituting oxidation rate constant кfrom (6) to (7), the following relationship is obtained for numerical evaluation of chromium diffusion coefficient:
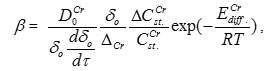
where “o” subscript is used to designate corresponding experimental data taken from [12].
Conversion of mass flow rate to the film growth rate would give its experimental values of 0.7 .10-7 μm/s.Usually, results of metallographic examination show that ΔCCrst is within 1-2 wt.%, and ΔCr does not exceed few micrometers.
Taking into account the above comments, numerical value of DCr = DCro exp (ECrdiff/RT) at 6000Сwould be ~ 10-13 cm2/s. Further on, taking Ediff. ≈ 240 kJ/mole value as typical for chromium diffusion in chromium steels, DCro ≈ 30 cm2/s or 3·109 µm2/s values can be determined, which in the first approximation could be further substituted to equation (7).
After that, the equation can be simplified and transformed as follows:
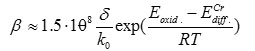
for evaluation of degree of the oxide films enrichment with chromium.
Kinetics of various steels oxidation, as well as coefficients of chromium diffusion in these steels may significantly vary, however, since ECrdiff >Eoxid. the exponent numeric values are less than one, these abruptly decreasing with temperature decrease. For instance, for chromium diffusion in iron, activation energy value is 465 kJ/mole [13]. Owing to this, with typical temperature dependences of oxidation rates ~200 kJ/mole, ~ 1·10-16 exponential term value can be obtained for 6000С temperature, and even ~ 1·10-18 – for 5000С. Therefore, oxide film enrichment with chromium is unlikely with the real steel oxidation rates at low temperature.
In this context, the situation with chromium diffusion in the real steels becomes more favorable, since significant effect of various alloying additives on diffusion mobility of this element in γ – iron has been experimentally determined. For instance, nickel, niobium, stannum and titanium additives in the amount of 1 at.% decrease Ediff.of chromium, respectively, by 7, 18, 32 and 41%. Silicon additive turned out to be most effective, because it almost halves activation energy value [12] – down to 236 kJ/mole level [13].
The above data concerning fairly strong effect of various alloying elements on diffusion mobility of chromium in the steels shows that the reliable prediction of the possibility of auto-protection effect manifestation for any specific steel requires experimental determination of this parameter just for this steel.
Possibilities of using proposed analytical relationships for obtaining additional information on the oxidation process
If the precise value of the rate of diffusion saturation of chemical reaction front with chromium is unknown, then the equation (3) derived above cannot be used for solving the inverse problem of evaluation of the reaction front movement velocity on the basis of the results of the further metallographic examination of the oxide films. However, this equation would be helpful for obtaining relative characteristics of its behavior, i.e. evaluating degree of oxidation rate decrease on the basis of data on the oxide films composition change.
As an example, let us evaluate the decrease of oxidation rate of 12%-chromium steel required for changing the oxide film composition, for instance, from Fe2.1Cr0.9O4 (СCr ~ 20 wt.%.) corresponding to the oxide composition without its additional enrichment with chromium, to FeCr2O4 oxide (СCr ~ 45 wt.%.).
We proceed from the premise that diffusion mobility of chromium atoms in the steel is kept constant in the course of oxidation, and densities of the initial and final oxides are equal, respectively, to P ≈5.0 g/cm3 and P = 4.79g/cm3 Then, substituting numerical values of parameters for each oxide composition to the relationship (3), it can be easily demonstrated that the oxidation rate at the moment of formation of this oxide composition should be reduced about 18 times with regard to that in the beginning of the enrichment process.
This reduction can be achieved by increasing the total thickness of the oxide film, or by improving its quality. In particular, if, for instance, the film thickness is already equal to 10 µm by the beginning of chromium content rise in the film and if the above composition is formed with 30 µm film thickness (this meaning only three times rate reduction for this reason), then the conclusion can be made on qualitative improvement of the protection properties of the film caused by its enrichment with chromium.
Oxide film enrichment with silicon and its effect on the film protection properties
Application of relationship (3) to the other alloying elements would also make it possible to evaluate the possibility of the oxide film enrichment with these elements. In this context, silicon is of special interest, because silicon additives to the steel increase its corrosion resistance. However, mechanism of this effect is still unclear. The most common opinion remaining now is that this is caused by the buildup of SiO2 thermodynamically stable oxides [2], which form thin protection layers.
It should be noted that the similar statements concerning formation of Cr2O3protection layers are also rather commonly used as the explanations of fair protection properties of the oxide films on chromium containing steels.
As regards silicon, indeed, many experiments carried out with various silicon containing steels show enrichment of the oxide films with this element, this being yet more proof of the point of its high diffusion mobility in the steels.
In particular, measured pattern of silicon distribution in EP 823 steel specimen held in lead at 6500С during 5000 hours is presented in [14]. Silicon concentration in the enrichment areas measured in this study does not exceed 4
wt.% value, this corresponding to the following enrichment degree:
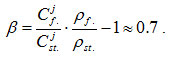
On the other hand, substitution of corresponding data for silicon oxide (CSi ~ 47 wt.%, Poxid =2.3g/cm3) to relationship (3) would give enrichment values for this oxide equal to β˜6 and β ˜8 for Si content in the steel equal, respectively, to 2 wt.% and 1.5 wt.%, this showing apparent insufficiency of β numerical value calculated on the basis of experimental data for the buildup of solid layer of SiO2 oxide.
Authors of [14] explained this contradiction by that the silicon concentrations measured by them characterized values averaged over the ion beam cross section, this not excluding presence of areas with much higher silicon concentration, which can be revealed using thinner beams.
However, assuming presence of such oxide film layers with significantly higher silicon concentration, their thickness should be: δ<< 1µm in order to eliminate its effect on the general nature of silicon distribution with total film thickness of a few micrometers. On the other hand, proceeding from the above mechanism, the possibility of spontaneous formation of such layer gives rise to strong doubts [1].
In [14], as well as in many other publications devoted to the studies on the steels relatively low-enriched with silicon and determination of silicon distribution over the oxide film thickness, it was noticed that its max concentration was usually observed in the vicinity of the film boundary with the steel matrix. In spite of significant enrichment of the oxide films with silicon βmax ~(2-3) no improvement of their protection properties was observed.
According to our estimates, under these conditions (the similar enrichment level), silicon may directly influence the oxidation process by formation of the corresponding oxide with noticeable decrease of oxidation rate, if only silicon concentration in the steel is at the level of ~ (3.5 – 4.5 wt.%) and higher.
This can be implicitly confirmed by the data on the results of studies focused mainly on the evaluation of the effect of silicon contained in the steels on their mechanical properties [15]. For this purpose, the following steel types were studied: T-91 (СCr ~ 9 wt.%, CSi ~ 0.2 wt.%.), T-91Si (СCr ~ 8 wt.%, CSi ~ 1.45 wt.%), and EP 823 (СCr ~ 11.7 wt.%, CSi ~ 1.1 wt.%), as well as two special steels: 2439 (СCr ~ 11.6 wt.%, CSi ~ 2.75 wt.%), and 2440 (СCr ~ 13.5 wt.%, CSi ~ 4.83 wt.%), which were exposed to both gas atmosphere and lead-bismuth. It was revealed in the course of the studies that after holding T-91 and EP 823 steel specimens in oxygen-saturated coolant at 5500Сduring 1500 hours, oxide films of the similar thickness (respectively, ~ 19 µm and ~ 20 µm) and composition (chrome spinel + magnetite) were formed on the specimens surface. This gave evidence of equability of these steels corrosion rates, this being adequately explainable, proceeding from the above statements, which show (see relationship 7) that auto-protection effect cannot be achieved in some cases.
On the other hand, oxide films of much less thickness were formed under the same conditions on 2439 and 2440 steel specimens enriched with silicon. Specific values of films thickness were not indicated in this publication, however it was noticed that it was impossible to measure their micro-hardness because of their small thickness.
Data from [16] can serve as straightforward evidence of direct involvement of silicon in formation of protective layer: among various steels, SX steel (СCr ~ 17.6 wt.%, CNi ~ 17.6 wt.% CSi ~ 4.8 wt.%) was tested in oxygen-saturated lead-bismuth at 5500С during 3000 hours. As a result, thin silicon based oxide film (δ~ 1 µm) was formed on the steel surface preventing corrosion progress under these conditions, while the other steels were corroded to various extents.
As it has been mentioned above, based on the available experimental data, commonly held opinion exists that, in general, steels with rather small silicon additives ~ (1.5 – 2 wt.%) have, nevertheless, higher corrosion resistance in HLMC as compared to that of similar additive-free steels.
Denying predominantly the possibility of direct involvement of silicon as a basis for the separate oxide layer assuring significant reduction of the oxidation rate of such steels, an alternative standpoint on its role in the oxidation processes can be proposed.
This standpoint is that the positive influence of small silicon additives is caused not by their direct but indirect effect on the oxidation process by means of increase of chromium additive diffusion mobility in the steel mentioned above. This additive is present in chromium steel in sufficient amount, has required thermodynamic properties and, hence, is the basic element in the oxide film capable, under the above certain conditions, of improving its protection properties.
Summary
Mechanism of alloying components delivery to the oxide films formed on the structural steels in heavy liquid metal coolants (oxide film enrichment with alloying components) is considered. Auto-protection of steels against corrosion is related to these components.
Formulae are proposed for numerical evaluation of degree of the oxide film enrichment with the alloying components, taking into account kinetics of the oxidation process and diffusion properties of steel with regard to the alloying component.
Influence of various factors on the process of the oxide films enrichment with alloying components has been studied.
Numerical evaluations of limitary and feasible characteristics of enrichment of steels used in HLMC with chromium and silicon were made, and the possibilities of using proposed analytical relationships were demonstrated.
Contradictory data on silicon effect on protection properties of the oxide films was analyzed, and hypothesis was proposed for the explanation of its positive influence on corrosion properties of chromium containing steels. This is that there is indirect effect by silicon on the oxidation process by means of increasing diffusion mobility of chromium in these steels.
References
- A.F. Gurbich, and S.L. Molodtsov, Application of IBA techniques to silicon profiling in protective oxide films on a steel surface, Nuclear Instruments and Methods in Physics Research, 226, 2004
- A.Ye. Rusanov, O.E. Levin, et al., Study on corrosion resistance of the fuel element claddings made of EP 823 steel after tests in flowing Pb-Bi, Paper presented at the Fourth Conference “Heavy Liquid Metal Coolants in Nuclear Technologies (HLMC-2013)”, Obninsk, 2013
- D. Joung, High Temperature Oxidation and Corrosion of Metals. Oxford, 2008
- F. Barbier, and A. Rusanov, Corrosion behavior of steels in flowing lead-bismuth, Journal of Nuclear Materials 296, 2001
- http://metallicheckiy-portal.ru/articles/chermet/fazovie_sostoyania/diffuzionnie_processi/2
- J. Moreau, Journal de Corrosion et Anticorrosion,1954, v.4, p.11
- J. Vanden Bosch, G. Coen, P. Hosemann, and S.A. Maloy, On the LME susceptibility of Si enriched steels, Journal of Nuclear Materials, 429, 2012
- L. Martinelli, T. Dufrenoy, et al., High temperature oxidation of Fe-9Cr-1Mo steel in stagnant liquid lead-bismuth at several temperatures and for different lead contents in the liquid alloy, Journal of Nuclear Materials, 376, 2008
- O. Yeliseyeva and V. Tsisar, Effect of temperature on the interaction of EP823 steel with lead melts saturated with oxygen.// Material Science. Vol.43. No. 2. 2007. P. 230-237.
- O. Yeliseyeva, V. Tsisar, and G. Btnamati, Influence of temperature on interaction mode of T91 and AISI316L steels with Pb-Bi melt saturated by oxygen, Corrosion Science, 50, 2008
- O. Yeliseyeva, V. Tsisar, V.M. Fedirko, and Ya.S. Matychak, Changes in the phase composition of an oxide film on EP-823 steel in contact with a static lead melt.// Fiz.-Khim. Mech. Mater. Vol. 40. No. 2. P. 90-98. 2004.
- O. Yeliseyeva, V. Tsisar, Zhou Zhangjian, Corrosion behavior of Fe-14Cr-2 and Fe-9Cr-2W ODS steels in stagnant liquid Pb with different oxygen concentration at 550 and 650 C.// Journal of Nuclear Materials. 442. 2013. P. 434-443.
- Q. Shi, J. Liu, H. Luan, et al., Oxidation behavior of ferritic/martensitic steels in stagnant liquid LBE saturated by oxygen at 6000C, Journal of Nuclear Materials, 457, 2015
- S.V. Salayev, P.N. Martynov, K.D. Ivanov, and O.V. Lavrova, Evaluation of diffusion induced release of metal components from structural steels exposed to heavy liquid metal coolants, Paper presented at the Conference “Heavy Liquid Metal Coolants in Nuclear Technologies (HLMC-2003)”, Obninsk, 2003
- Y. Kurata, M. Futakama, and S. Saito, Comparison of the corrosion behavior of austenitic and ferritic/martensitic steels exposed to static liquid Pb-Bi at 450 and 5500C, Journal of Nuclear Materials, 343, 2005
- Yu.V. Shumov, I.N. Gorelov, and Yu.A. Fyodorov, Temperature influence on growth rate of the oxide film caused by oxidation of ferritic/martensitic steel in the flowing lead-bismuth eutectic, Nuclear Power Engineering, No.3, 2004, pp.130 – 140.

This work is licensed under a Creative Commons Attribution 4.0 International License.









