Comparative evaluation of acute toxicity of nanoparticles of zinc, copper and their nanosystems using Stylonychia mytilus
Elena Rusakova1, Dianna Kosyan1-2, Elena Sizova1-2, Sergey Miroshnikov2 and Olga Sipaylova1
1Orenburg State University, Russia,460018, Orenburg, Pobedy pr. 13 2State Research Institution «All-Russian Research Institute of Beef Cattle Breeding», Russia, 460000, Orenburg, 9 Yanvarya Street, 29. Corresponding Author E-mail: elenka_rs@mail.ru
DOI : http://dx.doi.org/10.13005/ojc/31.Special-Issue1.13
Article Received on :
Article Accepted on :
Article Published : 07 Nov 2015
Nanotechnology has revolutionized the world by introducing a unique class of materials and consumer goods in many areas. This led to the production of new materials and devices. Nanotechnology has become an important priority in many countries. Despite its unique advantages and applications in everyday life and industry, the use of materials with nanometer dimensions raised a question of their safety for consumers and the environment [1]. The rapid propagation of various nanomaterials is a dilemma for regulatory authorities in relation to their hazard identification [2]. Recently, it has been written a lot about the urgent need to develop rules for nanomaterials. Such rules are needed for legislators to protect people from potentially adverse effects. As for manufacturers these rules are necessary in order to prove that nanoproducts must be produced carefully and with caution to avoid possible negative effects. However, it is turned out to be difficult to develop such rules [3]. At present, many efforts are made to use relatively simple nano-structured materials, such as metal oxide nanoparticles and carbon nanotubes in the content of high-strength materials, self-cleaning surfaces and stain-resistant textiles and for energy storage and conversion. [4] The study of more complex nanomaterials will lead to the use of these data in medical diagnostics and treatment, and modern electronics [5]. Within the past decade nanomaterials are used on a new commercial scale, it increases risks of possible toxic effects of nanoparticles that enter the environment because their potential toxicity for aquatic organisms is high [6]. Toxic effect of nanoparticles for aquatic ecosystems is associated with their physical and chemical properties and their action in aqueous media where dissolution, aggregation and agglomeration may occur [7]. Despite the fact that the production of these particles increased considerably in recent years, there are little data on their toxicity. Results of toxicity differ mainly due to the different methodology [8]. Different biological tests were discussed; they have been successfully used to assess the environmental impact of pollutants on invertebrates, algae and bacteria. Now they are more often used for risk assessment of nanoparticles on different levels of the aquatic food web [9]. Environmental risks and methodological approaches for testing the toxicity of nanomaterials using aquatic organisms were considered in recent publications [10]. For example, the toxicity of CuO and ZnO nanoparticles was assessed using a simplified model of the aquatic food chain in order to determine the bioaccumulation of toxic effects and particle transport through the trophic levels [11]. The experiment of using bacteria, protozoa, yeast, rotifer, algae, nematodes, crustaceans, fish, and amphibians as test objects in wastewater toxicological studies was described[12]. Only individual effects of nanoparticles were studied in most works. Despite the implementation of these data as a tool for assessing risks to the aquatic environment, there are still not enough data to fully understand the toxic effects of nanoparticles on aquatic organisms [13].
KEYWORDS:Stylonychia; mytilus; nanoparticles; zinc; copper; cell growth inhibition; cytotoxicity
Download this article as:| Copy the following to cite this article: Rusakova E, Kosyan D, Sizova E, Miroshnikov S, Sipaylova O. Comparative evaluation of acute toxicity of nanoparticles of zinc, copper and their nanosystems using Stylonychia mytilus. Orient J Chem 2015;31(Special Issue1). |
| Copy the following to cite this URL: Rusakova E, Kosyan D, Sizova E, Miroshnikov S, Sipaylova O. Comparative evaluation of acute toxicity of nanoparticles of zinc, copper and their nanosystems using Stylonychia mytilus. Orient J Chem 2015;31(Special Issue1). Available from: http://www.orientjchem.org/?p=12415 |
Introduction
Objectives
The aim of this study was to determine the toxicity of different doses of nanoparticles of zinc, copper and their compounds and the use of Stylonychia mytilus as a model organism.
Materials and Methods
Fresh water Stylonychia mytilus (wild strain) was utilised in the experiments during its exponential growth phase. The tested functions were survival and quantity (biomass). The cells were cultured on the medium of Lozina-Lozinskii with the addition of yeast (Saccharomyces cerevisiae) into the growing medium: NaCl-0,1%; KCl-0,01%; CaCl2-0,01%; MgCl2-0,01%; NаНСО3-0,02%. The concentrated medium was dilution with distilled water.
Cells at a stable growth rate were incubated at 20 ± 2°C. The particles were added to the cell culture for 24 hours. The quantity of cells was recorded after 1, 6, 12 and 24 hours. The amount of cells was determined utilising a light microscope (MT 5300L). The sensitivity of S. mytilus to the toxic particle was determined based on the time of the cell death, detected by a termination in movement of the protozoa together with cell membrane lysis. The amount of S. mytilus in 5 ml medium (without the addition of the particles) was utilised as a control for all experiments.
The nanoparticles utilized in the study are described in table 1.
Table 1: Characteristics of the utilised nanoparticles
|
Name |
Size [nm] | Phase and chemical composition | Production method | Surface (m2/g) |
Producer |
| Zn | 90 | 90%, the rest | the electric explosion | 5,34 | «Advanced |
| sorbing gases, zinc | of wire in an argon | powder | |||
| oxide and H2O | atmosphere | technologies», Russia | |||
| Cu | 97 | crystal copper 96,0 ± | high-temperature | 24 | «Advanced |
| 4,5%, copper oxide | condensation with | powder | |||
| – 4,0 ± 0,4% | subsequent | technologies», | |||
| modification of oxygen | Russia | ||||
| CuZn(alloy) | 65 | the electric explosion | 5-6 | «Advanced | |
| 60% copper and | of wire in an argon | powder | |||
| 40%zinc | technologies», | ||||
| atmosphere | Russia | ||||
| Cu-Zn (mixture) | 96,5 | 60% copper and | Gas-phase | 10 | «Advanced powder |
| 40%zinc | technologies», Russia |
Nanoparticles preparation was conduct in isotonic solution with an ultrasonic disperser (f-35 kHz, N 300 W), by dispersing for 30 minutes. The size of the particles was determined by a Field Emission Scanning Electron Microscope (JSM 740 IF). The concentration of the toxic particles varied between 6 ·10-6 M and 3.2 M.
ANOVA statistical analysis was utilized and then using the Tukey test (SPSS вер. 17.0). Differences were considered significant if p< 0.05.
Results and Discussion
Analysis of the data revealed different effects of nanoparticles of zinc, copper and nanosystems simplest cell culture (Table 2).
Table 2: Effects of nanoparticles of zinc, copper and their compounds in cell culture S. mytilus
|
Concentration, mМ |
Time of exposition: 1 hour |
Time of exposition: 6 hour |
||||||
|
Zn |
Cu |
CuZn (alloy) |
Cu-Zn (mixture) |
Zn |
Cu |
CuZn (alloy) |
Cu-Zn (mixture) |
|
|
3,2 |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
|
1,6 |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
|
0,8 |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
|
0,4 |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
|
0,2 |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
|
0,1 |
LC50 |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
|
0,05 |
LOEC |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
|
0,025 |
LOEC |
Tox |
Tox |
Tox |
LC50 |
Tox |
Tox |
Tox |
|
0,0125 |
NOEC |
Tox |
Tox |
Tox |
LOEC |
Tox |
Tox |
Tox |
|
0,00625 |
NOEC |
Tox |
Tox |
Tox |
LOEC |
Tox |
Tox |
Tox |
|
0,003 |
NOEC |
Tox |
Tox |
Tox |
NOEC |
Tox |
Tox |
Tox |
|
0,0015 |
NOEC |
Tox |
Tox |
Tox |
NOEC |
Tox |
Tox |
Tox |
|
0,00078 |
NOEC |
LC50 |
Tox |
Tox |
NOEC |
Tox |
Tox |
Tox |
|
0,00039 |
NOEC |
LOEC |
Tox |
Tox |
NOEC |
LC50 |
Tox |
Tox |
|
0,00019 |
NOEC |
LOEC |
LC50 |
Tox |
NOEC |
LOEC |
Tox |
Tox |
|
9х10-5 |
NOEC |
LOEC |
LOEC |
Tox |
NOEC |
LOEC |
LC50 |
Tox |
|
4х10-5 |
NOEC |
NOEC |
LOEC |
LC50 |
NOEC |
LOEC |
LOEC |
Tox |
|
2х10-5 |
NOEC |
NOEC |
LOEC |
LOEC |
NOEC |
NOEC |
LOEC |
LC50 |
|
1х10-5 |
NOEC |
NOEC |
LOEC |
LOEC |
NOEC |
NOEC |
LOEC |
LOEC |
|
6х10-6 |
NOEC |
NOEC |
NOEC |
NOEC |
NOEC |
NOEC |
NOEC |
NOEC |
|
Time of exposition: 12 hour |
Time of exposition: 24 hour |
|||||||
|
Concentration, mМ |
Zn |
Cu |
CuZn (alloy) |
Cu-Zn (mixture) |
Zn |
Cu |
CuZn (alloy) |
Cu-Zn (mixture) |
|
3,2 |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
|
1,6 |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
|
0,8 |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
|
0,4 |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
|
0,2 |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
|
0,1 |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
|
0,05 |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
|
0,025 |
LC50 |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
|
0,0125 |
LOEC |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
|
0,00625 |
LOEC |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
|
0,003 |
NOEC |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
Tox |
|
0,0015 |
NOEC |
Tox |
Tox |
Tox |
LC50 |
Tox |
Tox |
Tox |
|
0,00078 |
NOEC |
Tox |
Tox |
Tox |
LOEC |
Tox |
Tox |
Tox |
|
0,00039 |
NOEC |
LC50 |
Tox |
Tox |
LOEC |
Tox |
Tox |
Tox |
|
0,00019 |
NOEC |
LOEC |
Tox |
Tox |
NOEC |
Tox |
Tox |
Tox |
|
9х10-5 |
NOEC |
LOEC |
LC50 |
Tox |
NOEC |
LC50 |
Tox |
Tox |
|
4х10-5 |
NOEC |
LOEC |
LOEC |
Tox |
NOEC |
LOEC |
Tox |
Tox |
|
2х10-5 |
NOEC |
NOEC |
LOEC |
LC50 |
NOEC |
LOEC |
LC50 |
Tox |
|
1х10-5 |
NOEC |
NOEC |
LOEC |
LOEC |
NOEC |
LOEC |
LOEC |
Tox |
|
6х10-6 |
NOEC |
NOEC |
NOEC |
NOEC |
NOEC |
LOEC |
LOEC |
LC50 |
Note. Tox – the concentration causing 0-39 % survival object; LC50 – the concentration causing 50% survival of the object; LOEC – the concentration causing 40-69 % survival object; NOEC – the concentration causing 70-100 % survival object. (P. Jackson, N. Raun Jacobsen, A. Baun, R. Birkedal, D. Kühnel, K. Alstrup Jensen, U. Vogel, H. Wallin Bioaccumulation and ecotoxicity of carbon nanotubes // Chemistry Central Journa, 2013. 7(1):154)
Toxic effect of initial nanoparticles and their nanosystems is specific and depends on the concentration and exposure time of the cells.
The object of the study was to compare the nanoparticles of copper, zinc and their composites, and subsequently determine the maximum toxic effect. In the course of the experiments it was found that nanoparticles of zinc (Zn), copper (Cu) and alloy (CuZn), mixture (Cu-Zn) have a similar effect on the cell of the test organism.
Table 3 shows the morphological changes in the cells (flocculation, rupture, atrophy), depending on the length of incubation, cell culture Stylonychia mytilus a toxicant.
Table 3: Morphological changes of cells S. mytilus on the background of toxic effects of nanoparticles
| Time of |
Cell morphology |
|||
| incubation, h |
Zn |
Cu |
CuZn (alloy) |
Cu-Zn (mixture) |
|
1 |
Normal |
Normal, |
Normal, |
Normal, |
|
flocculation |
flocculation |
flocculation |
||
|
2 |
Flocculation |
Flocculation, |
Flocculation |
Flocculation |
|
rupture |
||||
|
6 |
Flocculation, |
Flocculation, |
Flocculation, |
Flocculation, |
|
rupture |
rupture, atrophy |
rupture |
rupture |
|
|
12 |
Flocculation, |
Rupture, |
Rupture, |
Rupture, |
|
rupture |
atrophy |
atrophy |
atrophy |
|
|
Flocculation, |
Flocculation, |
Flocculation, |
Flocculation, |
|
|
24 |
rupture,atrophy |
rupture, atrophy |
rupture, atrophy |
rupture, atrophy |
Moreover, the localization of nanoparticles in the cells was dependent on the incubation time of Stylonychia mytilus and toxicant. Thus, peripheral distribution of the nanoparticles was observed in the cells after 1 and 6 hours of incubation. After 12 hours of incubation uniform distribution of nanoparticles in the cell was observed.
The results demonstrate no effect of the nanoparticles in the first hour of incubation and complete destruction of the membrane beginning from 6 hours of incubation. During the evaluation of time periods it was registered that the maximum toxic effect of nanoparticles on S. mytilus is observed after 24 hours of incubation.
The analysis of concentration effects has shown that Zn nanoparticles are less toxic (LC50 0.0015M) than Cu nanoparticles and their composites (CuZn (alloy), Cu-Zn (mixture)) (9 * 10-5 LC50 and LC50 2 * 10-5 M, LC50 6 * 10-6 M) (Figure 1).
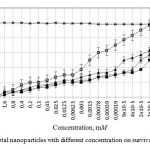 |
Figure 1: Influence of metal nanoparticles with different concentration on survival of Stylonychia mytilus Click here to View figure |
Study of toxic effects of nanoparticles has showed that all test samples had various toxic effects on cells of the test culture (Table 4).
Table 4: Biological effects of nanoparticles on Stylonychia mytilus
|
Name |
Concentration, mM |
|||
|
Тох |
LC50 |
LOEC |
NOEC |
|
|
Metals |
||||
|
Cu |
3,2 – 0,00019 |
9×10-5 |
4×10-5 – 2×10-5 |
1×10-5 – 6×10-6 |
|
Zn |
3,2 – 0,003 |
0,00015 |
0,00078 – 0,00039 |
0,00019 – 6×10-6 |
|
CuZn (alloy) |
3,2 – 4×10-5 |
2×10-5 |
1×10-5– 6×10-6 |
– |
|
Cu-Zn (mixture) |
3,2 – 2×10-5 |
1×10-5 |
6×10-6 |
– |
Note. Tox – the concentration causing 0-39 % survival object; LC50 – the concentration causing 50% survival of the object; LOEC – the concentration causing 40-69 % survival object; NOEC – the concentration causing 70-100 % survival object.
Cells of S. mytilus absorb nanoparticles of Zn, Cu, CuZn (alloy), Cu-Zn (mixture) and store them in digestive vacuoles. However, nanoparticles of Zn, Cu and their composites (CuZn (alloy), Cu-Zn (mixture)) inhibit the growth of cells and thus cause acute toxicity in the process of endocytosis and exocytosis. This fact inhibited cell growth even after 6 hour incubation and induced significant cytotoxic effects. Toxic effects of nanoparticles enhanced after 24 hours of incubation in connection with the inhibition of cell growth and increase of cytotoxicity of particles. After shorter time periods (1 hour) no changes were observed, the cells of protozoa remained active (Figure 2).
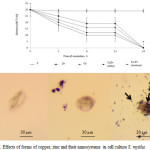 |
Figure 2: Effects of forms of copper, zinc and their nanosystems in cell culture S. mytilus Click here to View figure |
Figure 1. A) Changes in survival (mean ± SD) of S. mytilus through time upon treatment with Cu-Zn (mixture); B) S. mytilus one hour after the addition of the nanosystem Cu-Zn (mixture); C) S. mytilus 6 hours after the addition of the nanosystem Cu-Zn (mixture); D) S. mytilus 24 hours after the addition of the nanosystem Cu-Zn (mixture). The arrows show disruptions of the membrane. (Scale bar 30 µm).
Growth inhibition and subsequent cell death are connected with the fact that, first of all, the adsorption of nanoparticles occurs on the cell surface, and then they link to the cell membrane and penetrate into cell. Studies have shown that nanoparticles are generally localized in digestive vacuoles (lysosomes), wherein the cell is trying to digest or secrete them into the environment.
The mechanism of nanoparticles penetrating into cell (“Trojan horse effect “) is widely described [14]. It is related to the fact that partially soluble nanoparticles of metal oxides penetrate into cells, but in a different ionic form. The oxidative stress is considerably higher than in the case of penetrating ions of the respective metals, the transfer is controlled [15]. Indeed, after metal oxide nanoparticles have entered the cell, they may dissolve more than structural units of cell releasing harmful concentrations of metal ions in the cytoplasm. For example, the release of cytotoxic amounts of Cd has been shown for CdSe quantum dots under physiological conditions [16]. Moreover, due to the high surface area, nanoparticles adsorb heavy metals, polycyclic aromatic hydrocarbons, quinoline. Mackevica, A. et al. [17] demonstrated that toxicity of phenanthrene for Daphnia Magna increased by 60% in the presence of C60 aggregates. Thus, nanoparticles act not only as transfer vectors in the environment, but they also promote the penetration of contaminants adsorbed in nanoparticles into the cells of organisms, potentiating the toxic effects [18].
Nanoparticles can traverse even the strongest biological barriers such as the blood-brain barrier [19]. For example, Oberdörster E. et al. [20, 21] have shown that influence of fullerenes (C60) caused oxidative damage in the brain of the fish. Despite this, until recently the nanoscale materials were considered as variations of technical material and, thus, individual registration was not required [22].
So far, the fate and behavior of nanoparticles in the aquatic environment is an important question in terms of their impact on the environment and potential toxicity. The comparative evaluation of the toxic effect of silicone dioxide (SiO2) and titanium dioxide (TiO2) on marine microalgae Dunaliella tertiolecta was perfomed. It was found that the change of the dose-response and population growth of D. tertiolecta algae depends on the presence of nanoparticles TiO2. These particles influenced on 50% of the population. SiO2 nanoparticles were less toxic than TiO2 for D. tertiolecta. General toxic effect was established due to the contact between aggregates of nanoparticles and cell surfaces. SiO2 nanoparticles have a direct effect on the integrity of the cell membrane on the fourth day of exposure, while TiO2 nanoparticles exert their toxic effects in the first hours of exposure, resulting in mobile capture and agglomeration [23].
According to data obtained by Jagadeesh, E. et al. exposures of nanoparticles (iron oxide, cadmium sulfide nanocomposite and silver sulfide, cadmium sulfide and silver sulfide nanoparticles) caused lipid peroxidation and ROS, and suppressed the antioxidant defense system, such as catalase, glutathione reductase and superoxide dismutase of a model organism Mougeotia sp. The adsorption of nanoparticles on the surface of algae and membrane damage were confirmed through microscopic evaluation and increase of the protein content in the extracellular medium [24].
Conclusion
Thus, researches on nanoparticles safety are absolutely essential to support sustainable development of nanotechnology. Additional studies are needed because of the heterogeneity of the developing eco-toxicity of metal nanoparticles. The performed experiments indicate that specific biosensors (Stylonychia mytilus) that are highly sensitive to the toxic effect of metal nanoparticles (for example, Zn, Cu nanoparticles and CuZn (alloy), Cu-Zn (mixture) play a key role for the standardization of ecotoxicity of heavy metal nanoparticles.
Acknowledgements
This study was supported by the Russian Science Foundation, Russia. No. 14-36-00023
References
- Sajid, M., M. Ilyas, C. Basheer, M. Tariq, M. Daud, N. Baig, F. Shehzad, Impact of nanoparticles on human and environment: review of toxicity factors, exposures, control strategies, and future prospects / Environ Sci Pollut Res Int. 2015, 22(6): 4122-4143.
- Notter, D. A., D. M. Mitrano, B. Nowack, Are nanosized or dissolved metals more toxic in the environment? A meta-analysis / Environ Toxicol Chem. 2014, 33(12): 2733-2739.
- Moreno-Garrido, I., S. Pérez, J. Blasco, Toxicity of silver and gold nanoparticles on marine microalgae / Mar Environ Res. 2015, 16. pii: S0141-1136(15)00075-6.
- Hood, E., Nanotechnology: looking as we leap. Environ Health Perspect, 2004, 112: A740-A749.
- Bernstein, D., V. Castranova, K. Donaldson, B. Fubini, J. Hadley, T. Hesterberg, et al., Testing of fibrous particles: short-term assays and strategies. Inhal Toxicol, 2005, 17: 497-537.
- Donaldson, K., V. Stone, C. L. Tran, W. Kreyling, P. J. Borm, Nanotoxicology. Occup Environ Med, 2004, 61: 727-728.
- Goodman, C. M., C. D McCusker, T. Yilmaz, V. M. Rotello, Toxicity of gold nanoparticles functionalized with cationic and anionic side chains.Bioconjug Chem, 2004, 15: 897-900.
- Cheng, Y. S, G. K. Hansen, Y. F. Su, H. C. Yeh, K. T. Morgan, Deposition of ultrafine aerosols in rat nasal molds. Toxicol Appl Pharmacol, 1990, 106: 222-233.
- Kelly, J. T., B. Asgharian, Nasal molds as predictors of fine and coarse particle deposition in rat nasal airways. Inhal Toxicol, 2003, 15: 859-875.
- Baggs, R. B., J. Ferin, G. Oberdorster, Regression of pulmonary lesions produced by inhaled titanium dioxide in rats. Vet Pathol, 1997, 34: 592-597.
- Bermudez, E., J. B. Mangum, B. A. Wong, B. Asgharian, P. M. Hext, D. B. Warheit, et al., Pulmonary responses of mice, rats, and hamsters to subchronic inhalation of ultrafine titanium dioxide particles. Toxicol Sci, 2004, 77: 347-357.
- Donaldson, K., W. MacNee, Potential mechanisms of adverse pulmonary and cardiovascular effects of particulate air pollution (PM10). Int J Hyg Environ Health, 2001, 203: 411-415.
- Donaldson, K., D. Brown, A. Clouter, R. Duffin, W. MacNee, L. Renwick, et al., The pulmonary toxicology of ultrafine particles. J Aerosol Med, 2002, 15: 213-220.
- Ferin, J., G. Oberdorster, D. P. Penney, Pulmonary retention of ultrafine and fine particles in rats. Am J Respir Cell Mol Biol, 1992, 6: 535-542.
- Ferin, J., Pulmonary retention and clearance of particles. Toxicol Lett, 1994, 72: 121-125.
- Kuschner, W. G., H. Wong, A. D’Alessandro, P. Quinlan, P. D. Blanc, Human pulmonary responses to experimental inhalation of high concentration fine and ultrafine magnesium oxide particles. Environ Health Perspect, 1997, 105: 1234-1237.
- Mackevica, A., Skjolding, L.M., Gergs, A., Palmqvist, A., Baun. A. Chronic toxicity of silver nanoparticles to Daphnia magna under different feeding conditions. Aquat Toxicol., 2015, 161:10-6.
- MacNee, W., K. Donaldson, How can ultrafine particles be responsible for increased mortality? Monaldi Arch Chest Dis, 2000, 55:135-139.
- Wichmann, H. E., C. Spix, T. Tuch, G. Wolke, A. Peters, J. Heinrich, et al., Daily mortality and fine and ultrafine particles in erfurt, germany part I: role of particle number and particle mass. Res Rep Health Eff Inst, 2000, 5-86.
- Oberdorster, G., Pulmonary effects of inhaled ultrafine particles. Int Arch Occup Environ Health, 2001, 74 :1-8.
- Oberdorster, G., E. Oberdorster, J. Oberdorster, Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect, 2005, 113: 823-839.
- Oberdorster, G., R. M. Gelein, J. Ferin, B. Weiss, Association of particulate air pollution and acute mortality: involvement of ultrafine particles? Inhal Toxicol, 1995, 7: 111-124.
- Manzo, S., S. Buono, G. Rametta, M. Miglietta, S. Schiavo, G. Di Francia, The diverse toxic effect of SiO<sub>2</sub> and TiO <sub>2</sub> nanoparticles toward the marine microalgae Dunaliella tertiolecta / Environ Sci Pollut Res Int. 2015, 10: 45-69.
- Jagadeesh, E., Khan, B., Chandran, P., Khan, S.S. Toxic potential of iron oxide, CdS/Ag‚ S composite, CdS and Ag‚ S NPs on a fresh water alga Mougeotia sp. Colloids Surf B Biointerfaces,2014, 125, 284-90.

This work is licensed under a Creative Commons Attribution 4.0 International License.









