Acetylation of 2-Methoxynaphtalene with Acetic anhidryde over Zr4+-Zeolite beta
Edy Cahyono1, Sigit Priatmoko and Sri Haryani
Department of Chemistry, Universitas Negeri Semarang, Indonesia. Correspondence Author Email: edkim_unnes@yahoo.co.id
DOI : http://dx.doi.org/10.13005/ojc/31.Special-Issue1.09
Article Received on :
Article Accepted on :
Article Published : 05 Sep 2015
Regioselective acetylation of 2-methoxy naphthalene (2-MN) to 2-acetyl-6-methoxy naphthalene (2,6-ACMN) is the important step in the synthesis of pharmaceutical compounds such as (S)-naproxen. The liquid phase acetylation of 2-MN with acetic anhydride over Zr4+-zeolite beta catalyst was investigated under controlled reaction conditions. Catalyst was prepared by ion exchange and impregnation method. Zr4+-zeolite beta(ie) was obtained by ion exchange that done with 0,5 M ZrCl4 then calcined at 550 oC for 3 h and Zr4+-zeolite beta(ip) was obtained by impregnation method. Catalytic activity of catalysts was affected by preparing method, temperature, and reaction solvent. Catalyst that prepared by impregnation showed inactive on acetylation of 2-MN. 1-Acetyl-2-methoxynaphtalene (1,2-ACMN), 1-acetyl-7-methoxy-naphtalene (1,7-ACMN), and 2-acetyl-6-methoxy naphthalene (2,6-ACMN) were identified as the products of 2-MN acetylation over Zr4+-zeolite beta(ie) without solvent. Activity and selectivity of Zr4+-zeolite beta(ie) was increased in dichloromethane solvent at 140oC for 36 h, 1,8-ACMN was not identified and ratio of 1,2-ACMN to 2,6-ACMN was 1,6:1
KEYWORDS:Acetylation; 2-Acetyl-6-methoxynaphthalene; Zeolite beta; Regioselectivity; Solvent effect
Download this article as:| Copy the following to cite this article: Cahyono E, Priatmoko S, Haryani S. Acetylation of 2-Methoxynaphtalene with Acetic anhidryde over Zr4+-Zeolite beta. Orient J Chem 2015;31(Special Issue1). |
| Copy the following to cite this URL: Cahyono E, Priatmoko S, Haryani S. Acetylation of 2-Methoxynaphtalene with Acetic anhidryde over Zr4+-Zeolite beta. Orient J Chem 2015;31(Special Issue1). Available from: http://www.orientjchem.org/?p=10582 |
Introduction
Acetylation reaction is mostly done in the chemical industry to synthesize a variety of organic compounds. In many cases the acetylation reaction is not easy to obtain the desired product if there is more than one group in the starting material which can undergo the same reaction. To produce the desired product required special conditions that minimize byproducts. One of the factors that can determine the selectivity of the reaction is the catalyst. Drug class nonsteroidal anti-inflammatory (NSAID) has analgesic effects, antipyretic and anti-inflammatory. Drugs of this class is very effective to reduce the symptomatic pain. From a variety of NSAIDs, each has advantages and disadvantages which looks at the effects of therapeutic and side effects. (S)-Naproxen, or (+)-2-(6-methoxynaphtyl)-propionic acid, is a nonsteroidal anti-inflammatory drug that was introduced under the trade name Synex in 1976. In industry, these compounds were synthesized in four stages, starting with the Friedel-Crafts acylation of 2-methoxyinaphtalene for 2-acetyl-6 methoxyinaphtalene (2,6-ACMN), which is then converted to acid by Willgerodt reaction. Methylation produces a racemic mixture of an acid (R,S) which can be solved efficiently with sinkonidin , to obtain (S)-naproxen (Harrington and Lodewijk , 1997) . This process has several interesting problems. First, Friedel-Crafts acylation is not regiospesifik, and in addition not only 2,6-ACMN formed but also 1,2-ACMN isomer obtaioned, which must be separated by crystallization. Second, a significant amount of aluminum hydroxide and hydrochloric acid waste. Third, a number of undesirable reagents used, such as nitrobenzene as solvent acylation, ammonium sulfide for Willgerodt reaction, sodium hydride and methyl iodide. Furthermore, using of AlCl3 in stoichiometric amounts. These problems were combined with the toxicity and trouble some job work procedure, not in accordance with the ongoing process technology (Hoelderich, 1993) . Therefore, studies with alternative procedures which continue to be made more environmentally friendly. Friedel-Crafts acylation reactions are traditionally carried out with reagent AlCl3/chloride acid, alternative procedure that developed is the use of heterogeneous catalysts with acid anhydride. Some examples of heterogeneous catalysts for the synthesis of fine chemicals and intermediates are functionalised polymers such as zeolites or Amberlist A15 or Nafion (Heinichen and Hoelderich, 1999; Heidekum, Harmer, and Hoelderich, 1998; Heidekum, Harmer, and Hoelderich, 1999). Preparation of Zr4+-zeolite beta through ion exchange showed that ion exchange increases the surface area, pore volume but lower pore spokes compared the same parameters on the zeolite beta before treatment. Increase in the concentration of Zr4+ ion exchange in general increase the surface area and pore volume solids , but less pore jejeri show a clear trend . In general, ion exchange porosity results better than impregnation (Cahyono, 2011). Pore size and acidity properties of the resulting catalyst will have a good selectivity for the acetylation of 2-methoxy naphthalene.
The acetylation of 2-MN has been investigated over MCM-41, H-Y, ZSM-12 and BEA, and ZSM-5 and mordenite (Andy et al. 2000), The products are usually obtained, 1,2-ACMN and 2,6-ACMN. Schuster and Holderich (2008) showed that in the aceylation reaction the Fe3+– ion-exchanged zeolite beta to a conversion of acetic anhydride of 97%. The selectivity to the desired 2,6-ACMN was 37.4% after 24 h reaction time. A slightly higher selectivity to 2-acyl-6-methoxynaphthalene of 40% at 97% conversion could be achieved in presence of Cu2+ ion-exchanged BEA zeolite. The silver ion-exchanged zeolite gave a selectivity of 53% at 67% conversion. After 350 h reaction time the Cu2+ and the Fe3+ ion-exchanged zeolite led to complete conversion at a selectivity of 93% while the silver ion-exchanged zeolite deactivated after 48 h reaction time. Cahyono et al. (2010) has been investigated the activity and stereoselectivity of modified zeolite beta on cyclisation-acetylation of (R)-(+)-citronellal. The affect of catalyst preparing method, temperature, mol ratio of reactant, and solvent on the acetylation of 2-MN with acetic anhydride over Zr4+-zeolit beta were studied.
Material and Method
Zeolite beta was obtained from Tosoh (zeolite 940 NHA) with ratio of SiO2/Al2O3 37. Precursor ZrCl4, solvents toluene and dichloromethane, acetic anhydride were provided by EMerck. Characterization of catalysts was conducted by using pyridyn adsorption -IR method at energy laboratory LP2M ITS and morphology of the catalyst by SEM at Physics Laboratory UM. The reaction was performed in a reflux apparatus with cooler and nitrogen gas flow. Product acetylation analysis was done by GC and GC-MS.
Preparation and Characterization of Catalysts
Catalyts was prepared by impregnation method and ion exchange method. Preparation of Zr4+-zeolite beta(ip) catalyst by impregnation method was adapted from the procedure Yongzhong et al. (2004). At 1.0 g of zeolite beta was added 10 % of Zr4+ with 0.5 M zirconium chloride and then stirred for 24 hours. After drying at 100 ° C, the samples calcined at 550 oC for 3 hours, with a low heating rate of 1 ° C / min. Preparation of catalyst by ion exchange method was done by soaking 1 g of zeolite beta in 25 mL of 0.5 M ZrCl4 and then stirred for 24 hours, filtered until free of chloride ions. After drying at 100 ° C, the sample calcined at 550 oC for 3 h. Acidity of catalysts was determined by pirydin adsorbtion-FTIR method and morphology of catalysts was performed by using scanning electron micrograph (SEM).
In a 3-neck flask equipped with a magnetic stirrer and a cooler was added acetic anhydride (0.95 mL, 10 mmol) , 0.5 g of catalyst, and 20 mL of toluene and then stirred for 2 min, the mixture was added 1.58 g (10 mmol) of 2-methoxy naphthalene, the mixture heated at 70 0C under N2 gas flow. During the reaction 1 ml sample was taken after 12 hours, 24 hours and 36 hours. Then added 6 mL of distilled water and 3 ml n-hexane and separated by centrifuge. The organic phase was dried by adding of anhydrous Na2SO4, the products were analyzed by GC. The same procedure was done at difference temperature of 140 oC. Reactions also repeated with dichloromethane solvent, without solvent, and increasing amount of acetic anhydride.
Results and Discussion
Acidity of Catalysts
Pyridine have been used as probe molecules for the quantitative analysis of surface acidity of modified zeolite beta catalysts by FTIR spectroscopy. A scale of acidity and an evaluation of the relative acid strength were obtained for both Brønsted- and Lewis-acid sites. Table 1 shows the results of acidity analysis by pyridine adsorption-FTIR analysis.
Table 1: Acidity modified zeolite beta with Pyridine adsorption-FTIR analysis
| No | Sample | Parameter | Results (mmol/g) |
| 1 | Zeolite beta | Lewis acid |
0,1892 |
| Bronsted acid |
– |
||
| Total acid |
0,1892 |
||
| 2 | Zr4+-Zeolite beta (ie) | Lewis acid |
0,2373 |
| Bronsted acid |
0,0388 |
||
| Total acid |
0,2761 |
||
| 3 | Zr4+-Zeolite beta (ip) | Lewis acid |
0,1108 |
| Bronsted acid |
0,0182 |
||
| Total acid |
0,1290 |
Low total acidity on zeolite beta showed that calcination of NH4+-zeolite beta was not perfect released of NH3 for converted into H-zeolite beta. Zr4+-Zeolite beta (ip) that prepared by impregnation with 10% Zr4+ shows lower total acidity than Zr4+-Zeolite beta (ie) that prepared by ion exchange. High concentration of ZrCl4 covered of pores and surface of zeolite beta affected the pyridine adsorption on the active sites solids cannot optimum. Better distribution of Lewis acidity and Bronsted acidity sites is shown by Zr4+-Zeolite beta (ie).
SEM Analysis
Figure 1 shows the SEM images of zeolite beta before and after adsorption of Zr4+. Comparing the images of virgin (Figure 1(a)) and modified zeolite beta (Figure 1(b)), the difference image of catalysts is not clear, but the surface of Zr4+-zeolite beta shows brighter. than zeolite beta.
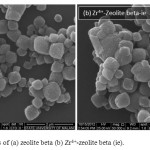 |
Figure 1: SEM images of (a) zeolite beta (b) Zr4+-zeolite beta (ie). Click here to View figure |
Existence of Zr4+ in the surface of Zr4+-zeolite beta (ie) was reported with XRF and XRD analysis (Cahyono, 2010)
Acetylation of 2-Methoxynaphtalene
Catalysts preparation method of the modified betazeolite influenced its catalytic activity on acetylation reactions. Both at 70 C and 140 oC, acetylation of 2-MN with acetic anhydride over Zr4+-zeolite beta(ip) and Zr4+-zeolite beta(ie) in toluene (mole ratio of acetic anhydride:2-MN, 1:1) showed almost no conversion of 2-MN after 24 h. Replacing the solvent and mole ratio of reactant showed better conversion.
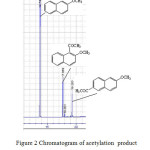 |
Figure 2: Chromatogram of acetylation product at 140 oC, 36 h in dichloromethane solvent Click here to View figure |
Activity of Zr4+-zeolite beta(ie) that showed with conversion of 2-MN of 29%. The selectivity to the desired 2,6-ACMN was 39% after 36 h reaction time. A slightly higher conversion to 2-acetyl-6-methoxynaphthalene could be achieved in dichloromethane solvent and added amount of acetic anhydride. Figure 2 shows the chromatograms of reaction product. Based on Schuster and Holderich (2008) report, reaction must be run in longer time for higher conversion.
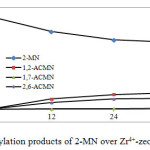 |
Figure 3: Acetylation products of 2-MN over Zr4+-zeolite at 140 oC. Click here to View figure |
Conclusion
Catalytic activity of catalysts was affected by preparing method, temperature, and reaction solvent. Zr4+-zeolite beta(ip) showed low activity on acetylation of 2-MN. Activity and selectivity of Zr4+-zeolite beta(ie) was increased in dichloromethane solvent at 140oC for 36 h, 1,7-ACMN was not identified and ratio of 1,2-ACMN to 2,6-ACMN was 1,6:1.
Reference
- Andy, P . Martinez, G., Lee, G., Gonzalez, H. Jones, C. W. and Davis, M. E., , Journal of Catalysis, 2000.,192: 215-223
- Cahyono, E., Muchalal, Triyono, Pranowo, HD, , Kinetic Study Cyclisation-acetylation (R)-(+)-Citronellal by Modified Natural Zeolite as Solid Solvent, Indonesian Journal of Chemistry, 2010., 10 (2)., 189-194.
- Cahyono, E.. Preparation and Characterisation of Zr4+-Zeolite Beta, Proceeding, National Seminar on Chemistry and Chemistry Education, UNS, Unnes, Undip, Unsoed. 2011
- Harrington, P.J. Lodewijk, E., , Org. Process Res. Dev. 1977.,72–76.
- Hoelderich W.F., Stud. Surf. Sci. Catal. 1993., 75: 127–136
- Heinichen, H.K., Hoelderich, W.F. , , J. Catal. 1999.,185 : 408–414.
- Heidekum, A., Harmer, M., Hoelderich, W.F., J. Catal. 1999., 181: 217–222.
- Heidekum, A., Harmer, M. , Hoelderich, W.F, , J. Catal. 1998., 176 : 260–263.
- Schuster, H. and Holderich W.F., , Applied Catalysis A: General2008., 350., 1–5
- Yongshong, Z., Yuntong, N., Jaenicke, S., dan Chuah, G.K., , Cyclisation of Citronellal over Zirconium Zeolite beta – a Highly Diastereoselective Catalyst to (±)-Isopulegol, J. Catal., 2004.,229.,404-413.

This work is licensed under a Creative Commons Attribution 4.0 International License.









