The Synthesis, Characterization and Comparative Anticorrosion Study of Some Organotin(IV) 4-Chlorobenzoates
Hastin Kurniasih1,2, Muhamad Nurissalam1,3, Bambang Iswantoro1,4, Hapin Afriyani1, Hardoko Insan Qudus1 and Sutopo Hadi1,*
1Department of Chemistry, University of Lampung, Bandar Lampung, Indonesia 35145
2Public Senior High School 1, Pringsewu, Indonesia 35373
3Muhammadiyah 1 Senior High School, Metro, Indonesia 34111
4Public Senior High School 16, Bandar Lampung, Indonesia 35155.
Corresponding Author Email : sutopo.hadi@fmipa.unila.ac.id
DOI : http://dx.doi.org/10.13005/ojc/310467
Article Received on :
Article Accepted on :
Article Published : 22 Oct 2015
The synthesis of 3 compounds of a series of dibutyl(IV) di-4-chlorobenzoate, diphenyl(IV) di-4-chlorobenzoate and triphenyltin(IV) 4-chlorobenzoate have successfully been performed by reacting the dibutyltin(IV) dichloride, diphenyltin(IV) dichloride and triphenyltin(IV) chloride respectively via the dibutyltin(IV) oxide, diphenyltin(IV) dihydroxide and triphenyltin(IV) hydroxide with 4-chlorobenzoic acid. All compounds synthesized were well characterized by 1H and 13C NMR, IR and UV-Vis spectroscopies as well as based on the microanalytical data. The anticorrosion activity of these compounds were tested on Hot Roller Plate (HRP) mild steel in DMSO-HCl solution using potentiodynamic method. The results revealed that the triphenyltin(IV) 4-chlorobenzoate clearly showed the strongest inhibitor activity compared to the other derivatives, while diphenyltin(IV) compounds were better than that of dibutyltin(IV) analogous. The results reported here indicated that the optimal activity were depended on the ligand attached to the metal centre and might also be related to the number of carbon atoms present in the organotin(IV) used.
KEYWORDS:corrosion inhibitor; mild steel; organotin(IV) compounds; potentiodynamic
Download this article as:| Copy the following to cite this article: Kurniasih H, Nurissalam M, Iswantoro B, Afriyani H, Qudus H. I, Hadi S. The Synthesis, Characterization and Comparative Anticorrosion Study of Some Organotin(IV) 4-Chlorobenzoates. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Kurniasih H, Nurissalam M, Iswantoro B, Afriyani H, Qudus H. I, Hadi S. The Synthesis, Characterization and Comparative Anticorrosion Study of Some Organotin(IV) 4-Chlorobenzoates. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12066 |
Introduction
Corrosion is an electrochemical reaction occured naturally and can proceed by itself, therefore the corrosion cannot totally be stopped1. Its reaction can normally only be controlled or slow down its reaction rate to decrease the corrosion process2. One of the methods to slow down the corrosion process is by applying the inhibitor. Corrosion inhibitor is a chemical compounds which can protect or slow down the corrosion process2,3.
The organotin compound are interesting mostly due to their significant effect on the biological activity4,5. Their biological activities are fundamentally determined by the number and the nature of organic groups bound to the central Sn atom6. The nature of the anionic groups seems acting only as a secondary factor6,7. The current investigations on the coordinating properties of carboxylates toward organotin compounds have led to the isolation of some new organotin(IV) carboxylates and carboxylate derivatives which have shown some interesting biological activities such as antimicrobial8-11, antitumor and anticancer6,10-14, antifungal activity7,9,15,16, antiplasmodial17 and the latest development of these compounds has led the new finding as new anticorrosion inhibitor18-21, therefore the investigation of organotin(IV) as possible anticorrosion is very challenging, has been and is still attracting much attention18-21.
In the present work, we reported the anticorrosion inhibition study of some dibutyl, diphenyl- and triphenyltin(IV) 4-chlorobenzoates toward mild steel in DMSO-HCl solution.
Experimental
Materials and Characterization
All reagents used were AR grade. Dibutyltin(IV) dichlorides ([(n-C4H9)2SnCl2]), diphenyltin(IV) dichloride ([(C6H5)2SnCl2]), triphenyltin(IV) chloride ([(C6H5)3SnCl]), 4-chlorobenzoic acid were obtained from Sigma, water HPLC grade, sodium hydroxide (NaOH) and methanol (CH3OH) were JT Baker products, and were used without further purification.
1H and 13C NMR spectra were recorded on a Bruker AV 600 MHz NMR (600 MHz for 1H and 150 MHz for 13C). All experiments were run in DMSO-D6 at 298K. The number of runs used for 1H experiments were 32 with reference at DMSO signal at 2.5 ppm, while the 13C were 1000-4000 scans with the reference DMSO signal at 39.5 ppm. IR spectra in the range of 4000-400cm-1 were recorded on a Bruker VERTEX 70 FT-IR spectrophotometer with KBr discs. Elemental analyses (CHNS) were performed on Fision EA 1108 series elemental analyser. The UV spectra were recorded in the UV region and were measured using a UV- Shimadzu UV-245 Spectrophotometer. Measurements were performed in 1 mL quartz-cells. Solutions were prepared using methanol as the solvent with concentration of 1.0×10-4M.
Preparation of Organotin(IV) Chlorobenzoates
The organotin(IV) chlorobenzoates used in this work were prepared based on the procedure previously reported13-16,21,and was adapted from the work by Szorcsik et al.9. An example procedure in the preparation of dibutyltin(IV) dichlorobenzoate was as follows:
To 3.0383 g (0.01 mol) [(n-C4H9)2SnCl2] in 50 mL methanol was added 0.8 g (0.02 mol) NaOH. The reaction mixtures were stirred for about 45 minutes. Compound 2 was precipitated out as white solid, filtered off and dried in vacuo till they are ready for analysis and further reaction. The average yield was 2.3508 g (95 %).
To 0.37338 g (1.5 mmol) compound 2 in 50 mL of methanol was added with 2 mole equivalents of 4-chlorobenzoic acid (0.2505 g) and was refluxed for 4 hours at 60 – 70°C. After removal of the solvent by rotary evaporator, the produced compounds [(n-C4H9)2Sn(4-OOCC6H4Cl)2] were dried in vacuo until they are ready for analysis and further use for corrosion inhibition test. The average yields were more than ~ 90 %.
A similar procedure was also adapted in the preparation of diphenyltin(IV) and triphenyltin(IV) derivatives, [(C6H5)2Sn(OOCC6H4Cl)2] and [(C6H5)3Sn(OOCC6H4Cl)], respectively. For triphenyltin(IV) only one mole equivalent of the 4-chlorobenzoic acid was used.
Corrosion inhibitor measurement
Mild steel hard roll plate types were cut in 2 x 1 cm2 and were successively polished with abrasive paper starting from grid 240, 400, 600 and 800. After the surface of the mild steel were homogenous, the surface were thoroughly washed with distilled water, dilute HCl and finally with acetone. The surface area of each mild steel was then measured, weight out and stored in a vacuum desiccator until they were used.
The potentiostat used was ER644 Integrated Potentiostat eDAQ type. It has three electrode cells which is assembly consisted of a working electrode of mild steel (2 cm2 exposed are), a saturated AgCl as a reference electrode and a platinum counter electrode. After being assembled, they were immersed in corrosive medium as the electrolyte and connected to potentiostat. The first step was the measurement of rate inhibition without inhibitor, all three electrodes were immersed for 10 minutes in the electrolyte. The potential was set up with scanning rate of 0.5 mV/s. The current change occurred were measured and recorded, the data obtained were then processed to determine the potentiodynamic graph (η against ln |j|) in which the current corrosion density (Icorr0) and corrosion potential (Icorri) were obtained using Tafel extrapolation method21. The similar way was used in the measurement of inhibition rate with inhibitor of organotin(IV) chlorobenzoate. The concentrations of inhibitor used were 0, 10, 20, 40, 60, 80 and 100 mg/L. The data in each measurement were recorded and then the percentages of inhibition efficiency (the corrosion rate) were calculated based on Equation 1.
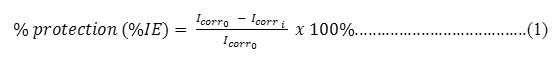
where %IE: the percentage of inhibition efficiency; Icorr0: current before the addition of inhibitor; Icorri: current after the addition of inhibitor
Results and Discussion
The synthesis of compounds of dibutyltin(IV) di-4-chlorobenzoate, [(n-C4H9)2Sn(4-OOCC6H4Cl)2] (3), diphenyltin(IV) di-4-chlorobenzoate [(C6H5)2Sn(OOCC6H4Cl)2] (6) and triphenyltin(IV) 4-chlorobenzoate, [(C6H5)3Sn(4-OOCC6H4Cl)] (9), were successfully performed from their chlorides [(n-C4H9)2SnCl2] (1), [(C6H5)2SnCl2] (4) and [(C6H5)3SnCl] (7), respectively, where all of the reactions in all cases were performed via [(n-C4H9)2SnO] (2), [(C6H5)2Sn(OH)2] (5) and [(C6H5)3SnOH] (8) respectively similar to those previously reported13-16, 21. The scheme 1 is an example of reaction step that occurred in the preparation of dibutyltin(IV) di-4-chorobenzate. The microanalytical data of all compounds synthesized are very good and all values obtained are in agreement to those of calculated values as shown in Table 1.
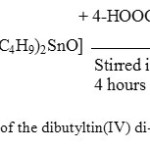 |
Figure 1: The scheme of preparative route of the dibutyltin(IV) di-4-chlorobenzoate Click here to View figure |
Table 1: The microanalytical data of the organotin(IV) compounds synthesized
|
Compound |
Elemental analysis found (calculated) |
|
|
C |
H |
|
| [(n-C4H9)2Sn(4–C6H4(Cl)COO)2] (3) |
48.2 (48.5) |
4.8 (4.8) |
| [(C6H5)2Sn(4–C6H4(Cl)COO)2] (6) |
53.2 (53.4) |
3.3 (3.1) |
| [(C6H5)3Sn(4–C6H4(Cl)COO)] (9) |
57.5 (57.6) |
3.8 (3.7) |
Some spectroscopy techniques were used in the characterizations of the compounds synthesized. The 1H and 13C chemical shifts of the compounds prepared are shown Table 2. A number of signals in the spectra recorded have been characterized carefully. The chemical shift (δ) of butyl protons attached to the tin metal appeared in the range of 0.92 ppm for Hδ up to 1.37-1.62 ppm for Hα and Hβ, and the carbons of butyl ligands are observed at position comparable with other similar compounds reported previously14,21,23. The chemical shift of phenyl protons attached to tin metal appeared in the range of 7.34 – 7.62 ppm, while the carbon of carboxyl group of all compounds as expected appeared in the region of 176 ppm14,21,23. The carbon atoms of the phenyl ligand as also expected appeared in δ of 131 – 126 ppm, while the carbons in the chlorobenzoate derivatives appeared in δ range of 140 – 130 ppm close to the reported values of similar compounds14, 21.
Table 2: 1H and 13C spectra of the compounds synthesized
|
Compound |
H in butyl or phenyl (ppm) |
H in benzoate (ppm) |
C in butyl, phenyl and benzoate (ppm) |
| [(n-C4H9)2Sn(4–C6H4(Cl)COO)2](3) |
Hα & Hβ:1.37-1.62 (m); Hγ: 1.29 (m); Hδ: 0.92 (t) |
7.34-7.86 (m) |
Cα: 21.3; Cβ: 26.6; Cγ: 25.9; Cδ: 14.2; C1: 174.9; C2: 139.3; C3 & C7: 129.7; C4 & C6: 128.6; C5: 125.1 |
|
[(C6H5)2Sn(4–C6H4(Cl)COO)2] (6) |
H2 & H6 7.58 (d, 4H); H3 & H5 7.48 (t, 4H); H4 7.36 (t, 2H) |
7.89 – 7.99 (m) |
C1-6 (phen): 131.7-126.9; C7: 175.9; C8: 139.8; C9: 130.4; C10: 164.9; C11: 129.7; C12: 129.0; C13: 130.2
|
|
[(C6H5)3Sn(4–C6H4(Cl)COO)] (9) |
H2 & H6 7.57 (d, 6H); H3 & H5 7.45 (t, 6H); H4: 7.31 (t, 3H) |
7.81 – 7.89 (d) |
C1-6 (phen): 131.1-126.2; C7: 175.4; C8: 139.3; C9 & C13: 130.1; C10 & C12: 128.7; C11: 128.2 |
Table 3: The characteristic and important IR bands of the organotin(IV) compounds (cm-1) synthesized.
| Compound |
3 |
6 |
9 |
References |
|
Sn-O |
434.6 |
591.1 |
765.50 |
800-400 |
|
Sn-O-C |
1029.2 |
1290.4 |
1243.3 |
1050-900 |
|
Sn-Bu |
674.9 |
– |
– |
740-660 |
|
CO2 asym |
1419.2 |
1596.7 |
1558.3 |
1600-1400 |
|
CO2 sym |
1558.2 |
1690.3 |
1631.6 |
1700-1550 |
|
C-H aliphatic |
2954 – 2860 |
– |
– |
2960 – 2850 |
|
Phenyl |
– |
1490.5; 725.7 |
1428.4; 729.4 |
1450, 730 |
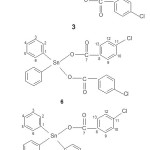 |
Figure 2: The proposed structure of some compounds synthesized and the suggested numbering of carbons in each compound Click here to View figure |
The important FT-IR data and their assignments are presented in Table 3. The characteristic band of the starting materials (1, 4, 7) is the presence of strong stretching band of Sn-Cl bond at 390 – 310 cm-1. In 1 for example, the Sn-Cl bond appeared at frequency of 334 .2 cm-1. The other characteristic bands of this compound appear as stretching band from butyl ligands at 1069 cm-1, and bending vibration of C-H aliphatic stretch of the butyl at frequency of 2956 – 2865 cm-1. When compound 1 is converted to compound 2, the main stretching band of Sn–Cl disappeared and a new strong band at frequency of 417.4 cm-1 appeared as one of the main stretching band. This band is characteristic for Sn–O bond in compound [(n-C4H9)2SnO] (2). The stretching band due to the butyls and their bending vibrations are still appearing as expected although the frequencies have little bit shifted. The formation of dibutyltin (IV) dichlorobenzoate compounds, [(n-C4H9)2Sn(OOCC6H4Cl)2], (3) is confirmed by the strong asymmetric stretching bands of the carboxylates which occurred at ca. 1400 cm-1 and the symmetric stretch at ca. 1600 cm-1 as well as the present of Sn-O stretching of the acid at 435 cm-1, and the appearance of these bands is confirming the success of the substitution reaction13-16,21.
The λmax of all the compounds obtained from the UV-Vis spectroscopy analyses is summarized in Table 4. It is clear that there was a shifting change in the lmax for each compound in any steps of the reaction. For example, the compound 1 has lmax of 210.7 nm, while compound 2 has λmax of 202.9 nm. This information gave an indication that there was a shift to a shorter λmax value when the conversion of compound 1 to 2 took place. The wave-length shift to a shorter lmax could occur due to either the solvent used in the measurement or the effect of an auxochrome of the ligand. However in this study, as the solvent used for all measurements was the same (methanol), the change in the λmax that occured must be due to the auxochrome effect13. In the case of compound 1 and 2, there is an oxide group which has electron drawing effect bigger in compound 2 than that of chloride group in 1. As a result, the electron transition in 2 is hard to occur. Thus, the measured λmax was getting shorter in compound 2 than in compound 124. Similar results are also observed for other changes as can be seen from Table 4. For example, in compound 3, the electron drawing effect of 4–C6H4(Cl)COOH is less than chloride in 1, so the electron transition in this molecule will be easier (the energy required is less), thus producing longer λmax, 285.6 nm.
Table 4: The λmax of the UV-Vis Spectra of the Organotin(IV) Compounds
| Compound |
λmax (nm) |
||
|
π– π* |
n- π |
Benzene ring |
|
| [(n-C4H9)2SnCl](1) |
– |
210.7 |
– |
| [(n-C4H9)2SnO](2) |
– |
202.9 |
– |
| [(n-C4H9)2Sn(4–C6H4(Cl)COO)2] (3) |
– |
285.6 |
– |
| [(C6H5)2Sn(4–C6H4(Cl)COO)2] (6) |
204.4 |
296.7 |
404.3 |
| [(C6H5)3Sn(4–C6H4(Cl)COO)] (9) |
207.2 |
301.5 |
406.9 |
In our previous studies on the antifungal, anticancer and anticorrosion activity of the compounds similar to the compounds reported here13-16,21, it has been shown that optimal activity of the antifungal, anticancer and anticorrosion has been associated with the number of carbon atoms of the ligand present in the organotin(IV) used13-16,21,24, where in general, the derivative of triphenyltin(IV) carboxylate which contain 18 carbon atoms has the highest activity13-16,21. The same phenomena interestingly werre also observed in this study.
As shown in Table 5, the derivatives of triphenyltin(IV) compounds showed the highest inhibition ability in the series, and the diphenyltin(IV) compound was stronger in inhibiting than that of dibutyltin(IV) compound. Thus the number of carbon atoms present as well as the type of the ligands has significant effect on the anticorrosion activity of the organotin(IV) compounds tested.
Table 5: The Percentage Inhibition Efficiency
| Compounds |
% IE |
| 4-chlorobenzoic acid |
-12.1 |
| [(n-C4H9)2SnCl2] |
21.9 |
| [(n-C4H9)2SnO] |
30.3 |
| [(n-C4H9)2Sn(4–C6H4(Cl)COO)2] (3) |
52.3 |
| [(C6H5)2Sn(4–C6H4(Cl)COO)2] (6) |
58.1 |
| [(C6H5)3Sn(4–C6H4(Cl)COO)] (9) |
64.3 |
It was also observed that the organotin(IV) chlorobenzoate compounds synthesized exhibited much higher corrosion inhibition compared to those of the ligands, starting materials and intermediate products. In this respect, our results are consistent with a well-known fact that many biologically active compounds become more active upon complexation than in their uncomplexed forms26.
Furthermore, the ligand used indeed increased the corrosion activity as shown by their %IE value which was negative. This can be understood that the hydrogen ion of the acid in the medium used increasing the number of hydrogen ion due to ionization occurred to it, as a result the ligand increased the corrosion toward the steel. According to Crowe27 the actual biological activity of diorganotin compounds of the type RR’SnXY (R and R’ = alkyl or aryl; X and Y= anions) is determined solely by the RR’Sn2+ moiety.
Conclusions
The organotin(IV) chlorobenzoate compounds were successfully prepared and it has been shown from the discussion above that they have shown some promising result to be used as anticorrosion inhibitor. The fact that triphenyltin(IV) chlorobenzoate derivative have shown the highest anticorrosion ability was in line with other data relating to the number of carbon atom present in the compound and might also relate to the ability of phenyl ligand to draw electron from the metal center as a result the metal became more positive and attached strongly to the steel and protect the steel from corrosion in the corrosive medium. Further studies are being carried to find the best explanation for this phenomenom.
Acknowledgments
The authors are grateful to Directorate of Research and Community Services, Directorate General of Higher Education, The Ministry of Research, Technology and Higher Education, Republic of Indonesia that provide fund for this project to be undertaken through Hibah Kompetensi (Competitive Research Grant) Scheme 2015 with contract number 162/UN26/8/LPPM/2015, 30 March 2015 . Thanks also go to Prof. Bohari M. Yamin, Universiti Kebangsaan Malaysia for helping in doing microanalysis and Prof. Dr. Hasnah Osman of School of Chemistry, University of Sains Malaysia for NMR experimentation.
References
- Uhlig, H.H. The Corrosion Hand Book. The Electrochemical Society, Inc, John Wiley & Sons, New York; 2000.
- Rieger, H.P. Electrochemistry, 2nd ed., Chapman and Hall Inc, New York, 412-442, 1992.
- Al-Mhyawi, S.R. Orient. J. Chem. 2014, 30, 541-552.
- Tiekink, E.R.T. Appl. Organomet. Chem. 1991, 5, 1-23.
- Shahid, K.; Ali, S.; Shahzadi, S.; Akhtar, Z. Turk. J. Chem. 2003, 27, 209-215.
- Pellerito, L.; Nagy, L., Coord. Chem. Rev. 2002, 224, 111-150.
- Bonire, J.J; Ayoko, G.A.; Olurinola, P.F.; Ehinmidu, J.O.; Jalil, N.S.N.; Omachi, A.A., Metal-Based Drugs 1998, 5, 233-236.
- Gielen, M. J. Braz. Chem. Soc. 2003, 14, 870-877.
- Szorcsik, A.; Nagy, L.; Gadja-Schrantz, K.; Pellerito, L.; Nagy, E.; Edelmann, E.T. J. Radioanal. Nucl. Chem. 2002, 252, 523-530.
- Gleeson, B.; Claffey, J.; Ertler, D.; Hogan, M.; Müller-Bunz, H.; Paradisi, F.; Wallis, D.; Tacke, M. Polyhedron 2008, 27, 3619-3624.
- Rehman, W.; Badshah, A.; Khan, S.; Tuyet, L.T.A. Eur. J. Med. Chem. 2009, 44, 3981-3985.
- Li, Y.; Li, Y.; Niu, Y.; Jie, L.; Shang, X.; Guo, J.; Li, Q. J. Bioinorg. Chem. 2008, 102, 1731-1735.
- Hadi, S.; Rilyanti, M. Orient. J. Chem. 2010, 26, 775-779.
- Hadi, S.; Rilyanti, M.; Suharso. Indo. J. Chem. 2012, 12, 172-177.
- Hadi, S.; Irawan, B.; Efri. J. Appl. Sci. Res. 2008, 4, 1521-1525.
- Hadi, S., Rilyanti, M.; Nurhasanah. Modern Appl. Sci. 2009, 3, 12-17.
- Hansch, C.; Verma, R.P. Eur. J. Med. Chem. 2009, 44, 260-273.
- Rastogi, R.B.; Singh, M.M.; Singh, K.; Yadav, M. Port. Electrochim. Acta. 2005, 22, 315-332.
- Singh, R.; Chaudary, P.; Khausik, N.K. Rev. Inorg. Chem. 2010, 30, 275-294.
- Rastogi, R.B.; Singh, M.M.; Singh, K.; Yadav, M. Afr. J. Pure Appl. Chem. 2011, 5, 19-33.
- Hadi, S.; Afriyani, H.; Anggraini, W.D.; Qudus, H.I.; Suhartati, T. Asian J. Chem. 2015, 27, 1509-1512.
- Evans, U.R. Oxidation and Corrosion of Metals, 3rd Ed. Arnold, London, 1968.
- Nath, M.; Yadav, R.; Gielen, M.; Dalil, H.; de Vos; D.; Eng, G. Appl. Organometal. Chem. 1997, 11, 727-736.
- Sudjadi, The Structure Determination of Organic Compounds. Ghalia Publishers, 1985. (In Indonesian).
- Chohan, Z.H.; Rauf, A. Synth. React. Inorg. Met.-Org. Chem. 1996, 26, 591-604.
- Gershon, H. J. Med. Chem. 1974, 17, 824-827.
- Crowe, A.J. The antitumour activity of tin compounds; in Gielen M., Ed. Metal-Based Drugs, Freund Publishing House, Freund, 1989, 1, 103-149.

This work is licensed under a Creative Commons Attribution 4.0 International License.









