The influence of parameters of spark discharge generator on dimensional characteristics of synthesized TiO2 nanoparticles
Alexey Efimov*, Valentin Sukharev, Victor Ivanov, Anna Lizunova
Department of Physical and Quantum Electronics, Moscow Institute of Physics and Technology, Dolgoprudny, Russia
Correspondence Author Email: efimov.aa@mipt.ru.
DOI : http://dx.doi.org/10.13005/ojc/310456
Article Received on :
Article Accepted on :
Article Published : 11 Nov 2015
A multi-spark discharge generator was used for the synthesis of TiO2 nanoparticles. The nanoparticles were obtained in the form of fractal-like agglomerates with an average size of 30-60 nm consisting of primary spherical nanoparticles with a diameter of about 7-8 nm according to TEM measurements. We found that changing the operating parameters of the generator - energy of the capacitor (2 to18 J), repetition frequency of discharge (0.5 to 4 Hz) and velocity of airflow (1.4 to 5.4 m/s) changed only the size of the agglomerates while the size of the primary nanoparticles stayed the same.
KEYWORDS:Titanium dioxide; nanoparticles; spark discharge; synthesis
Download this article as:| Copy the following to cite this article: Efimov A, Sukharev V, Ivanov V, Lizunova A. The influence of parameters of spark discharge generator on dimensional characteristics of synthesized TiO2 nanoparticles. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Efimov A, Sukharev V, Ivanov V, Lizunova A. The influence of parameters of spark discharge generator on dimensional characteristics of synthesized TiO2 nanoparticles. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12581 |
Introduction
The synthesis of nanoparticles by spark discharge is a new and promising method for obtaining small nanoparticles of high chemical purity1 with the size less than 10 nm2,3. The synthesis of nanoparticles by this method is a result of processes of evaporation and condensation of the vapor of the electrode material2,4. The method is simple and allows the synthesis of new phases and compounds5 using only electrical energy, gas and electrode material6. This method does not require solvents, surfactants and precursors6,7. The synthesis of nanoparticles by spark discharge has been used successfully to produce nanoparticles of metals2,3 and metal oxides8,9, carbon10 and semiconductor materials11. In recent years, nanoparticles obtained by spark discharge are being actively studied for their application as materials for hydrogen storage1,12, catalytic surface activation13,14, testing of filters15 and toxicological studies10,16. However, despite the progress achieved in the study of the obtained nanoparticles properties by spark discharge, there is still not enough data about the characteristics of the resulting nanoparticles and the influence of the synthesis parameters on their size. Therefore, the first goal of this study was the characterization of nanoparticles obtained in the process of electrical erosion of titanium electrodes in air. The second goal of this study is to investigate the influence of synthesis parameters – energy of the capacitor, the repetition frequency of discharge and the velocity of airflow on the dimensional characteristics of the resulting nanoparticles in a multi-spark discharge generator.
Experimental
Material and Methods
For the experiments we used a multi-spark discharge generator of aerosol nanoparticles17 in the mode with varied operating parameters: energy of the capacitor (W), repetition frequency of discharges (f) and air flow velocity (v) in ranges of 2-18 J, 0.5-4.0 Hz and 1.4-5.4 m/s, respectively. Its schematic diagram is shown in Fig. 1. The source electrodes were metal cylinders with a nominal diameter of 10 mm made of Ti (Fig. 1). Pulse energy release in the discharge gaps in the near-electrode zones led to the erosion of the electrode material with the subsequent formation of nanoparticles as a result of process of evaporation and condensation2. The electrode gaps were purged with a purified stream of dry air (Fig. 1).
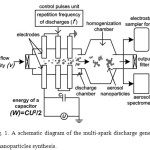 |
Figure 1: A schematic diagram of the multi-spark discharge generator for aerosol nanoparticles synthesis. Click here to View figure |
Sampling of the aerosol for the nanoparticles characterization was carried out using tubes connected to the aerosol spectrometer and the electrostatic sampler for collection of nanoparticles (Fig. 1). Measurements of size of the nanoparticles in the aerosol flow in real-time was performed by the aerosol spectrometer SMPS 3936 (TSI Inc.). Characterization of the collected nanoparticles was performed using transmission electron microscope (TEM) JEM-2100 (JEOL Ltd.), lattice parameters and structure of the particles were studied by the selected area electron diffraction (SAED). Elemental analysis of the particles was carried out using energy-dispersive X-ray spectroscopy (EDX).
Result and Discussion
Fig. 2 shows a TEM-image of the nanoparticles synthesized in the process of electrical erosion of titanium electrodes in air at the capacitor energy W of 2 J, the repetition frequency of discharges f of 0.5 Hz and the airflow velocity v of 5.4 m/s in the multi-spark discharge generator. It was found that the nanoparticles were fractal-like agglomerates with an average size dag of 30-60 nm, consisting of spherical primary particles with an average diameter dpr of 7-8 nm. The particle size distribution of the primary particles (Fig. 3a) and the agglomerates (Fig. 3b) is well described by functions of log-normal distribution, which is typical for the methods of evaporation and condensation18.
 |
Figure 2: TEM and SAED (inset) images of the nanoparticles synthesized in the process of electrical erosion of titanium electrodes in air at the following operating parameters: W=2 J, f=0.5 Hz and v=5.4 m/s. Click here to View figure |
 |
Figure 3: The particles size distribution for primary particles (a) and agglomerates (b), calculated from the TEM results. Nanoparticles synthesized by electrical erosion of titanium electrodes in air at W=2 J, f=0.5 Hz and v=5.4 m/s. Click here to View figure |
The elemental analysis of the nanoparticles revealed presence of two characteristic peaks responsible for the elements Ti and O in the composition (Fig. 4), indicating that the nanoparticles are in fact titanium oxide, which is justified as their synthesis occurred in an oxygen-containing air atmosphere.
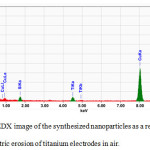 |
Figure 4: EDX image of the synthesized nanoparticles as a result, the electric erosion of titanium electrodes in air. |
The structural analysis established that synthesized nanoparticles had a crystalline structure with a tetragonal structure in the phase of anatase, which is typical for TiO2 in the nanoscale size dimension19. The inset to Fig. 2 shows the SAED with three intensive spot rings, which correspond to the largest characteristic interplanar spacings dhkl for the anatase phase and equal to 0.352, 0.237 and 0.188 nm for crystal planes (101), (004) and (200), respectively, and many weak rings.
The dependence of the average size of the agglomerates dag and primary particles dpr on the operating parameters of the generator – energy of the capacitor W, the repetition frequency of discharges f and the airflow velocity v in the corresponding ranges: 2-18 J, 0.5-4 Hz and 1.4 to 5.4 m/s is presented in Fig. 5. The average size of primary particles of the dpr is obtained from the TEM measurements and the average size of the agglomerates dag is determined by the aerosol spectrometer.
Fig. 5 shows that the size of the primary nanoparticles dpr is almost independent of the operating parameters of the generator within the TEM measurement uncertainty. This can be explained by the constancy of the cooling rate of the vapors due to the stability of relaxation of electro-discharge plasma20. Since the cooling rate of the vapors does not change, the primary particles are synthesized with a constant average size. On the contrary, the size of the agglomerates dag varies greatly depending on the operating parameters of the generator. For example, by increasing the airflow velocity v from 1.4 to 5.4 m/s the average size of the agglomerates dag was reduced almost 2 times – from 56 to 32 nm. Similar changes in the average size of agglomerates dag were observed with variation in W and f (Fig. 5). The dependence of the average size of the agglomerates dag from the operating parameters of the generator may relate to the process of agglomeration of aerosol nanoparticles due to the change of the nanoparticles concentration. Increase of the capacitor energy W increases the pulse energy in the discharge gaps, leading to an increase in the amount of product of the electrodes erosion. As a result, the concentration of the synthesized particles increases, which leads to the particle agglomeration, and consequently increases the average size of the agglomerates dag. Fig. 5 shows that with the increasing capacitor energy W from 2 to 18 J the average size of the agglomerates dag increases from 32 to 48 nm. Additional confirmation of the aerosol particles agglomeration can be seen from the dependence of the average size of the agglomerates dag on the repetition frequency of discharges f (Fig. 5). Increasing the repetition frequency of discharges f from 0.5 to 4 Hz the average size of the agglomerates dag increases from 32 to 41 nm. This result indicates intensification of the particles agglomeration because the increase in frequency also increases the amount of evaporated electrode material. The particles agglomeration during the synthesis by spark discharge can be reduced by the increase of the airflow, leading to the decrease in the aerosol particles concentration. Fig. 5 shows that with increasing airflow velocity v the average size of agglomerates is reduced. This fact should be considered in experiments for synthesis of weakly agglomerated nanoparticles, which are usually required in applications in electronics and ceramic and composite materials.
 |
Figure 5: Dependence of the average size of the primary particles dpr and the agglomerates dag on the operational parameters of the multi-spark discharge generation – the capacitor energy W, the repetition frequency of discharges f and the airflow velocity v. Click here to View figure |
Conclusions
The nanoparticles of TiO2 were synthesized by a multi-spark discharge generator. The nanoparticles were in the form of fractal-like agglomerates with an average size of 30-60 nm composed of primary spherical nanoparticles with a diameter of about 7-8 nm.
We found that the size of primary nanoparticles remained almost constant, when changing the operating parameters of the generator – the capacitor energy (2-18 J), the repetition frequency of discharges (0.5 to 4 Hz) and the airflow velocity v (1.4 to 5.4 m/s). On the other hand, the size of the agglomerates depended significantly on the operating parameters of the generator.
Decrease of the capacitor energy and the repetition frequency of discharges, and increase of the airflow velocity leads to the synthesis of smaller agglomerates.
Acknowledgements
This work was supported by a grant from the Russian Scientific Fund (project № 15-19-00190).
References
- Vons, V. A.; Anastasopol, A.; Legerstee, W. J.; Mulder, F. M.; Eijt, S. W. H.; Schmidt-Ott, A. Low-temperature Hydrogen Desorption and the Structural Properties of Spark Discharge Generated Mg Nanoparticles. Acta Mater. 2011, 59, 3070–3080.
- Tabrizi, N. S.; Ullmann, M.; Vons, V. A.; Lafont, U.; Schmidt-Ott, A. Generation of Nanoparticles by Spark Discharge. J. Nanoparticle Res. 2008, 11, 315–332.
- Byeon, J. H.; Park, J. H.; Hwang, J. Spark Generation of Monometallic and Bimetallic Aerosol Nanoparticles. J. Aerosol Sci. 2008, 39, 888–896.
- Swihart, M. T. Vapor-phase Synthesis of Nanoparticles. Curr. Opin. Colloid Interface Sci. 2003, 8, 127–133.
- Tabrizi, N. S.; Xu, Q.; Pers, N. M. van der; Schmidt-Ott, A. Generation of Mixed Metallic Nanoparticles from Immiscible Metals by Spark Discharge. J. Nanoparticle Res. 2009, 12, 247–259.
- Pfeiffer, T. V.; Feng, J.; Schmidt-Ott, A. New Developments in Spark Production of Nanoparticles. Adv. Powder Technol. 2014, 25, 56–70.
- Pfeiffer, T. V.; Kedia, P.; Messing, M. E.; Valvo, M.; Schmidt-Ott, A. Precursor-Less Coating of Nanoparticles in the Gas Phase. Materials 2015, 8, 1027–1042.
- Roth, C.; Ferron, G. A.; Karg, E.; Lentner, B.; Schumann, G.; Takenaka, S.; Heyder, J. Generation of Ultrafine Particles by Spark Discharging. Aerosol Sci. Technol. 2004, 38, 228–235.
- Kim, J.-T.; Chang, J.-S. Generation of Metal Oxide Aerosol Particles by a Pulsed Spark Discharge Technique. J. Electrost. 2005, 63, 911–916.
- Evans, D. E.; Harrison, R. M.; Ayres, J. G. The Generation and Characterisation of Elemental Carbon Aerosols for Human Challenge Studies. J. Aerosol Sci. 2003, 34, 1023–1041.
- Vons, V. A.; Smet, L. C. P. M. de; Munao, D.; Evirgen, A.; Kelder, E. M.; Schmidt-Ott, A. Silicon Nanoparticles Produced by Spark Discharge. J. Nanoparticle Res. 2011, 13, 4867–4879.
- Vons, V. A.; Leegwater, H.; Legerstee, W. J.; Eijt, S. W. H.; Schmidt-Ott, A. Hydrogen Storage Properties of Spark Generated Palladium Nanoparticles. Int. J. Hydrog. Energy 2010, 35, 5479–5489.
- Byeon, J. H.; Hwang, J. Fabrication of a Metal Membrane on a Perforated Polymer Substrate by Palladium Aerosol Activation and Subsequent Electroless Plating. Acs Appl. Mater. Interfaces 2009, 1, 261–265.
- Byeon, J. H.; Yoon, K. Y.; Jung, Y. K.; Hwang, J. Thermophoretic Deposition of Palladium Aerosol Nanoparticles for Electroless Micropatterning of Copper. Electrochem. Commun. 2008, 10, 1272–1275.
- Efimov, A. A.; Ivanov, V. V.; Volkov, I. A.; Subbotina, I. R.; Pershin, N. A. Filtration of Nanosized Particle Aerosols by Electret Fibrous Filters. Nanotechnologies Russ. 2013, 8, 789–798.
- Park, J. H.; Mudunkotuwa, I. A.; Kim, J. S.; Stanam, A.; Thorne, P. S.; Grassian, V. H.; Peters, T. M. Physicochemical Characterization of Simulated Welding Fumes from a Spark Discharge System. Aerosol Sci. Technol. 2014, 48, 768–776.
- Efimov, A. A.; Ivanov, V. V.; Bagazeev, A. V.; Beketov, I. V.; Volkov, I. A.; Shcherbinin, S. V. Generation of Aerosol Nanoparticles by the Multi-spark Discharge Generator. Tech. Phys. Lett. 2013, 39, 1053–1056.
- Wiley: Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles, 2nd Edition – William C. Hinds.
- Harano, A.; Shimada, K.; Okubo, T.; Sadakata, M. Crystal Phases of TiO2 Ultrafine Particles Prepared by Laser Ablation of Solid Rods. J. Nanoparticle Res. 2002, 4, 215–219.
- Bazelyan, E. M.; Raizer, Y. P. Spark Discharge; CRC Press, 1997.

This work is licensed under a Creative Commons Attribution 4.0 International License.









