Synthesis, characterization and electrical study of new bis-fused tetrathiafulvalenecontains functional groups
Meriam Boumedjout1, Amel Bendjeddou2,*, Tahar Abbaz1,2, SihamTiaouianine1, Lakhmici Kaboub1, Abdelkrim Gouasmia1 and Didier Villemin3
1Laboratory of Organic Materials and Heterochemistry, University of Tebessa, Algeria 2Laboratory of Aquatic and Terrestrial Ecosystems, University of Souk Ahras, Algeria 3Laboratory of Molecular and Thio-Organic Chemistry, ENSICAEN-University of Caen, France
Corresponding Author Email: bendjeddouamel@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310405
Article Received on :
Article Accepted on :
Article Published : 03 Dec 2015
New series of bis-fused tetrathiafulvalene contains functional groups was newly synthesized. The synthesis was carried out by using routes involving cross coupling, reduction, oxidation, and Wittig-type reactions. The identity of these new donors is confirmed by NMR1H, mass spectrometry, and elemental analysis. We have used the cyclic voltammetry in order to determine the character donors-π of these molecules and to verify the reversibility of the redox process involved. Molecular orbital diagram has been calculated using density-functional calculations. Charge transfer complexes with tetraflurotetracyano-quinodimethane (TCNQF4) were prepared by chemical redox reactions.
KEYWORDS:Tetrathiapentalene; Tetrathiafulvalene; Electrochemistry; Organic materials; Conductivity
Download this article as:| Copy the following to cite this article: Boumedjout M, Bendjeddou A, Abbaz T, Tiaouianine S, Kaboub L, Gouasmia A, Villemin D. Synthesis, characterization and electrical study of new bis-fused tetrathiafulvalenecontains functional groups. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Boumedjout M, Bendjeddou A, Abbaz T, Tiaouianine S, Kaboub L, Gouasmia A, Villemin D. Synthesis, characterization and electrical study of new bis-fused tetrathiafulvalenecontains functional groups. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12831 |
Introduction
The synthesis and characterization of new heterocyclic systems based on chalcogens (oxygen, sulfur, selenium, and tellurium) has been one of the central objectives in contemporary organic chemistry. Several interesting systems have been synthesized and characterized. Especially, sulfur-based heterocyclic systems have found widespread applications in modern material science and medicinal chemistry1.
Tetrathiafulvalenes and related heterocycles have received much interest due to their unique electron-donating capabilities2. A number of interesting properties of the TTF moiety includes its ability to form molecular metals and superconductors at low temperatures. It has also been incorporated in a number of macrocyclic systems for use as molecular sensors, enzyme biosensors, switches, wires and shuttles, exploiting the inherent electron donor properties3.
Organic materials that are capable of conducting electricity in the manner of semiconductors (e.g. silicon and germanium), conductors (e.g. copper and silver) and superconductors (e.g. very low temperature mercury) have drawn a huge amount of research interest over the past quarter century4,6. The versatility of organic chemistry, which enables subtle changes to be made in the molecular structure, introduces the possibility of ‘fine tuning’ the properties of these materials to suit a specific task. These organic materials are also potentially cheaper and easier to manufacture than traditional inorganic conductors. They may also be polymeric or crystalline and exist as solids or thin films, thus the potential for industrial applications is very large.
The suggestion that organic materials could exhibit electronic conductivity was advanced over eighty years ago7. Now the research on these substances remains extensive and multi-disciplinary, bringing together organic chemists, solid state physicists. X-ray crystallographers and materials scientists. These materials have the potential to make a comparable impact on the electronics industry in the twenty-first century, as silicon and other inorganic materials did in the twentieth.
A large proportion of this worldwide research effort, and many of its successes, have concerned the synthesis of electrically conducting charge-transfer (CT) and radical-ion (RI) salts. The work presented in this paperconcerns the synthesis of novel organic electron donor compounds and the synthesis and properties of the CT derived from them.
Experimental Section
General
NMR spectra were recorded on an NMR 400 WP apparatus (Bruker BioSpin GmbH, Silberstreifen 4, 76287Rheinstetten, Germany). FAB-MS spectra were recorded on a JOEL JMS-DX 300 spectrophotometer (JEOL Europe, Planet II, Gebouw B., Leuvensestreenweg 542, B-1930 Zaventem, Belgium). Uncorrected points of fusion were assessed with a Buchi 510 apparatus (BüchiLabortechnik AG, Meierseggstrasse 40, 9230Flawil, Switzerland). Cyclic voltammetry was carried out on a PAR-273 potentiostat/galvanostat (Alltest Instruments, Inc. 500 Central Ave. Farmingdale, NJ, USA). All computations were performed with the Gaussian 09 program package (Gaussian, Inc. 340 Quinnipiac St Bldg 40 Wallingford, CT, USA)8 using the 6-31G(d,p) basis set. Density functional theory (DFT) calculations were carried out using a B3LYP method (public field method). All the solvents were dried by standard procedures and commercial reagents were used without further purification. All reactions were carried out under an inert nitrogen atmosphere.
Synthesis and Characterization of 2,3-dipentylthio-8,9-dicarboxymethyl-tetrathiapentalene 4
Under a nitrogen atmosphere, 25 mL of freshly distilled triethylphosphite was added to the mixture of 1 (0.3 g, 0.60 mmol) or 2(0.31 g,0.60 mmol) and 3 (0.15 g,0.60 mmol). The resulting mixture was heated over an oil bath up to 110 °C and stirred for a further 12 h. The solvent was then removed under reduced pressure. Compound 6 was obtained by column chromatography of the residue (silica gel, eluting with dichloromethane and petroleum ether (2:1)) in 57% yield from 1 and 20% yield from 2, compound 4 as a brown powder. M.p.: 176°C. TLC: Rf = 0.64 (CH2Cl2). 1H NMR (400 MHz, CDCl3, δ, ppm):0.98 (t, 6H, CH3CH2); 1.35 (m, 8H, CH3CH2CH2); 1.66 (q, 4H, CH2CH2S); 2.80 (t, 4H, CH2S); 3.87 (s, 6H, CH3O). MS (NOBA, FAB > 0): 702 [M + H]+. Anal. calcd for C24H28O4S10: C, 41.11; H, 4.03; found:C, 40.91; H, 3.92.
Synthesis and Characterization of 2,3-dipentylthio-8,9-dihydroxymethyl-tetrathiapentalene5
Compound 4 (0,4g, 0,57mmol) was dissolved in dry THF (15 ml) to give a dark red solution. To this sodium borohydride (0,10 g, 2.64 mmol) and zinc chloride (0.73 g, 5.35 mmol) were added and the mixture heated under reflux for 3 hours, after which the mixture had turned dark yellow. The mixture was cooled to 0°C and ethylacetate (10 ml) added, followed by a dropwise addition of saturated aqueous ammonium chloride (5 ml). The mixture was then filtered, and dichloromethane added to the filtrate causing the immediate formation of a precipitate that was filtered off and washed with water to leave the product 5 as a dark green powder.Yield: 70%.M.p.: 242°C. TLC: Rf = 0.4 (AcOEt). 1H NMR (400 MHz, CDCl3,δ, ppm): 0,96 (t, 6H,CH3CH2); 1.33-1.47 (m, 8H, CH3CH2CH2);1.51-1.68 (m, 4H, SCH2CH2); 2.81 (t, 4H, SCH2); 3.8 (d, 4H, CH2OH); 5.32 (t, 2H, OH). MS (NOBA, FAB > 0): 646[M + H]+. Anal. calcd for C22H28O2S10: C, 40.96; H, 4.37; found:C, 40.81; H, 4.17.
Synthesis and Characterization of 2,3-dipentylthio-8,9-diformyl-tetrathiapentalene 6
To a solution of diol 5 (0.3 g; 0.46 mmol) in a THF (20 mL) /CH2Cl2 (20 mL) mixture at room temperature, was added MagtrieveTM (1.5 g). The reaction mixture was refluxed overnight, and the oxidizing agent was discarded by filtration. The filtrate was evaporated and purified by column chromatography using CH2Cl2 /petroleum ether (7/3) as the mixture of eluents to afford dialdehyde6 as black powder.Yield: 50%.M.p.: 270°C. TLC: Rf = 0.6 (CH2Cl2). 1H NMR (400 MHz, CDCl3,δ, ppm): 0.96 (t, 6H,CH3CH2); 1.33-1.47 (m, 8H, CH3CH2CH2);1.52-1.68 (m, 4H, SCH2CH2); 2.81 (t, 4H, SCH2); 10.20 (s, 2H, CHO). MS (NOBA, FAB > 0): 642[M + H]+. Anal. calcd for C22H24O2S10: C, 41.22; H, 3.77; found:C, 41.02; H, 3.60.
Synthesis and Characterization of bis[(pyridyl) ethenyl]-tetrathiapentalene 7-9
To a stirred solution of diformyl-tetrathiapentalene 6(240mg, 0.37 mmol: 1equiv) and triphenylheteroarylmethylphosphonium chloride (2.5 equiv) in dry toluene (30 mL) was added excess triethylamine (1 mL, 20 equiv). The reaction mixture was refluxed for 4h under nitrogen. After cooling, the product was subjected to column chromatography on silica gel (CH2Cl2and THF), affording the expected compounds 7-9 as powder.
2,3-dipentylthio-8,9-bis[2-(2-pyridyl)ethenyl]-tetrathiapentalene 7
Brown powder. Yield: 67%. M.p.: 238°C. TLC: Rf = 0.66 (THF). 1H NMR (400 MHz, CDCl3,δ, ppm): 0.97 (t, 6H,CH3CH2); 1.35-1.47 (m, 8H, CH3CH2CH2);1.52-1.68 (m, 4H, SCH2CH2); 2.81 (t, 4H, SCH2); 6.95 (d, 2Hethylenic, J = 15.75 Hz); 7.13 (t, 2Harom, J = 5.95 Hz); 7.30 (d, 2Harom, J = 7.80 Hz); 7.64 (t, 2Harom, J = 7.80 Hz); 7.66 (d, 2Hethylenic, J = 15.75 Hz); 8.57 (d, 2Harom, J = 4.60 Hz). MS (NOBA, FAB > 0): 792[M + H]+. Anal. calcd for C34H34N2S10: C, 51.61; H, 4.33; found:C, 51.32; H, 4.18.
2,3-dipentylthio-8,9-bis[2-(3-pyridyl)ethenyl]-tetrathiapentalene 8
Brown powder. Yield: 63%. M.p.: 247°C. TLC: Rf = 0.68 (THF). 1H NMR (400 MHz, CDCl3,δ, ppm): 0.97 (t, 6H,CH3CH2); 1.35-1.47 (m, 8H, CH3CH2CH2);1.52-1.68 (m, 4H, SCH2CH2); 2.81 (t, 4H, SCH2); 6.95 (d, 2Hethylenic, J = 15.75 Hz); 7.32 (d, 2Harom, J = 8.05 Hz); 7.66 (d, 2Hethylen, J = 15.75 Hz); 7.81 (t, 2Harom, J = 8.05 Hz); 8.52 (d, 2Harom, J = 4.85 Hz); 8.70 (d, 2Harom, J = 2.10 Hz). MS (NOBA, FAB > 0): 792 [M + H]+. Anal. calcd for C34H34N2S10: C, 51.61; H, 4.33; found:C, 51.30; H, 4.20.
2,3-dipentylthio-8,9-bis[2-(4-pyridyl)ethenyl]-tetrathiapentalene9
Brown powder. Yield: 57%. M.p.: 265°C. TLC: Rf = 0.7 (THF). 1H NMR (400 MHz, CDCl3,δ, ppm): 0.97 (t, 6H,CH3CH2); 1.35-1.47 (m, 8H, CH3CH2CH2);1.52-1.68 (m, 4H, SCH2CH2); 2.81 (t, 4H, SCH2); 7.03 (dd, 4Hethylenic); 7.31(dd,4Harom); 8.49 (dd,4Harom). MS (NOBA, FAB > 0): 792 [M + H]+. Anal. calcd for C34H34N2S10: C, 51.61; H, 4.33; found:C, 51.28; H, 4.23.
Results and Discussion
By a cross-coupling reaction of alkylthio tricyclic1 or 29-11and the commercially available dimethyl 1,3-dithiole-2-thione-4,5-dicarboxylate 3 with a large excess of triethylphosphite in refluxing toluene, the target molecule dimethyl 2-[5-{4,5-Bis(n-pentylthio)-1,3-dithiol-2-ylidene}-[1,3]dithiolo[4,5-d][1,3]dithiol-2-ylidene]-1,3-dithiole-4,5-dicarboxylate 4 was obtained as a brown solid in moderate yield.
Thereduction of thedicarboxymethyl-TTP4 with NaBH4 /ZnCl2in refluxing tetrahydrofuran (THF) leads to a 70% yield of the correspondingbis(hydroxymethyl)TTP5and subsequent smooth oxidation with MagtrieveTM reagent proved to be the best alternative strategy to reachbis(formyl) TTP6. The latter was obtained in 50% yield after separation from a mixture consisting in the monoformyl intermediate and various overoxidation products (Scheme 1).
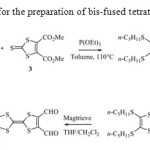 |
Scheme 1: Synthetic route for the preparation of bis-fused tetrathiafulvalene derivatives 4-6 |
In order to produce precursors of type’s pyridine exhibiting an extended π system a pyridine ring was incorporated into the diformyl-tetrathiapentalene 6, as substituent. The corresponding bis[(pyridyl)ethenyl]-tetrathiapentalene 7-9 were synthesized by using a Wittig type condensation involving the diformyl-tetrathiapentalene 6 and an appropriate phosphonium ylide. The later was generated in situ in refluxing acetonitrile in presence of an excess of triethylamine and of the corresponding phosphonium salt, respectively: the triphenyl(2-pyridylmethyl) phosphonium chloride (two equivalent), the triphenyl(3-pyridylmethyl) phosphonium chloride (2equiv) and the triphenyl(4-pyridylmethyl) phosphonium chloride (2 equiv) (Scheme 2).
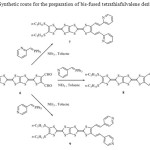 |
Scheme 2: Synthetic route for the preparation of bis-fused tetrathiafulvalene derivatives 7-9 Click here to View scheme |
According to the NMR coupling constant value found for the vinyl group (J = 15.75 Hz), a trans configuration can be attributed to the isolated compounds 7-9. This configuration is not surprising because it corresponds to a Wittig reaction performed from an ylide of stabilized type.
Electrochemical study
Cyclic voltammetry involves a linear sweep towards anodic potentials and then towards cathodic potentials (in the case of TTFs), for the range of potentials in which the product is oxidized and reduced. Peaks are observed, corresponding to the various stages of oxido-reduction12,13. If the species formed at the electrode remains stable over the time period of the analysis, allowing a passage backwards and forwards under tension, then the system is reversible.
We evaluated the reducing power of the new donors in solution by cyclic voltammetry to test whether they could readily be oxidized to generate new potentially conductive materials. This also allowed the stability of the oxidation states obtained (reversibility of redox systems and determination of oxidation potentials, as appropriate) to be tested.
The redox behavior of these new functional bis-TTF was studied in solution by cyclic voltammetry (CV). Measurements were performed under nitrogen at room temperature using a glassy carbon working electrode, a Pt counter electrode and a standard calomel electrode (SCE) as reference, with tetrabutylammonium perchlorate (n-Bu4 NClO4 , 0.1 M) in dry acetonitrile, as supporting electrolyte. A scan rate of 100 m·Vs−1 was used. The CV measurements showed reversible redox waves for all the compounds studied and the results of the corresponding oxidation potentials Eoxare summarized in Table 1.
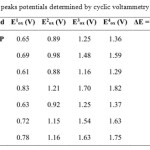 |
Table 1: Oxidation peaks potentials determined by cyclic voltammetry for compounds4-9 Click here to View table |
![Figure1.Voltammogramofbis[pyridyl)ethenyl]-tetrathiapentalene 7](http://www.orientjchem.org/wp-content/uploads/2015/12/Vol31_No4_Synt_Meri_Fig1-150x150.jpg) |
Figure 1: Voltammogram of bis[pyridyl)ethenyl]-tetrathiapentalene 7 |
Cyclic voltamograms of all the new unsymmetrically substituted donors consist of four pairs of one-electron redox waves. The first redox potentials of 4-9 are comparable to this of the corresponding tetrapentylthio-tetrathiapentalene (TTC5-TTP)14. These results strongly indicate that a positive charge formed by the first oxidation mainly distributes over the TTP moiety. In contrast, the second redox potentials of 6, 8 and 9 are higher than those of the correspondingTTC5-TTP probably due to occurrence of the second oxidation on the formyl or the pyridyl TTP moiety. As a result, the difference between the first and second redox potentials (ΔE = E2 −E1) of 6, 8 and 9 is larger by 0.19 V than that of TTC5-TTP. This suggests on-site Coulombic repulsion in the dication is enhanced compared with that in (TTC5-TTP)2+. In contrast, ΔE values of 4, 5 and 7 are comparable to this of TTC5-TTP. This result indicates that both the two positive charges formed by two successive oxidation processes significantly distributes on the TTP moiety.
Theoretical calculation
Band theory provides a vital insight into how crystalline materials behave when subjected to an electric field. Consider a large number (N) of identical atoms, far enough apart so their interactions are negligible. Every atom has the same energy level diagram. This energy level diagram shows the possible quantum states for the electrons and their associated energy levels. Each energy level is filled according to the Pauli exclusion principle (lowest levels filled first).
If the atoms are pushed together, because of the electronic interactions and the exclusion principle the energy levels change. Some move to higher energy and some move to lower energy. Each valence electron state for the system, formerly a sharp energy level that could accommodate N electrons, becomes spread out into a band containing N closely spaced levels. Since N is usually very large (circa 1023), the energy levels form a continuous distribution of energies within a band. The higher energy levels form the conduction band (LUMO), while the lower energy levels form the valence band (HOMO). The highest energy state in the valence band is the Fermi level, Ef. The size of the energy gap between these 2 bands governs the conducting ability in crystalline materials.
If the energy gap is large (3-6 eV) between a filled valence band and an empty conduction band the material is considered to be an insulator. If the energy gap is smaller (0.1-1.0 eV), the material is a semi-conductor. If there is overlap between the bands (as in a metal), the material is a conductor. In conjunction with these energy considerations, it is necessary for the material to possess partially filled bands to permit the electrons to move.
The extent of occupation of the energy levels and the size of the band gap regulate the electrical properties of a crystalline material. This means that target materials to be designed and synthesised need to possess partially filled delocalised bands, similar to those found in a metal. This situation arises on occasion when planar conjugated molecules crystallise in a stack as with charge transfer complexes. In these complexes, the transfer of electrons between donor and acceptor stacks provide the means for the formation of partially filled bands.
The energy of the frontier orbitals of the various products (4-9) was calculated according to density functional theory15,16 (DFT; base 6-31G, method B3LYP Figure 2). Based on the energy levels of the highest occupied molecular orbital (HOMO), compounds 5 and 7 were identified as the best donor molecules for the formation of TTF-TCNQF4 CTCs.
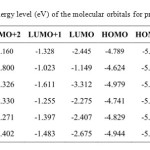 |
Table 2: Energy level (eV) of the molecular orbitals for products 4–9 |
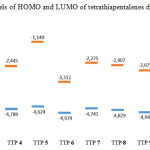 |
Figure 2: Levels of HOMO and LUMO of tetrathiapentalenes derivatives4-9 |
Preparation of the materials
Charge transfer complexes are a special case where metallic-like conductivities are obtained from essentially non-metallic, covalent, organic molecules. A C-T complex is formed by the interaction of an electron donor (D) and an electron acceptor (A). Electron donors are compounds with low ionization potential, while electron acceptors are compounds withhigh electron affinity. The donor and acceptor are bound together by an electrostatic attraction, not a chemical bond. Partial electron transfer between the donor molecule and the acceptor molecule generates this electrostatic attraction.
We used the direct synthesis method based on oxido-reduction in solution17-19to prepare the CTCs. The two components (the electron donor and acceptor) were dissolved separately in boiling acetonitrile and the two hot solutions were then mixed. The mixture was allowed to cool slowly and some of the solvent was allowed to evaporate, and we thereby isolated a solid with the characteristics of the expected complex.
The conductivity of materials is best measured with mono-crystal samples. In this situation, conductivity is dependent on the direction of the electric field with respect to the axes of the crystal. In general, the growth axis of the crystal, which corresponds to the axis along which the molecules are stacked, is the most conductive. However, in the absence of such mono-crystals, bars of compressed powder can be used for these measurements. This approach provides a mean value for the conductivity of the material. It should be noted, however, that bars of compacted powder are generally about an order of magnitude less conductive than single crystals.
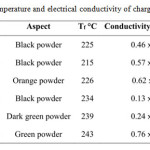 |
Table 3: Fusion temperature and electrical conductivity of charge transfer complexes Click here to View table |
All the materials synthesized were classified as semiconductors (table 3). The conductivity measured for a bar of compacted powder was 10-4 to 10-1 S.cm-1 for these materials. Values of conductivity 10 times higher could be anticipated for single crystals.
Conclusions
We herein describe the synthesis and the characterization of novel unsymmetrically substituted bis-fused tetrathiafulvalene bearing alkyl chains at one end of the π-electron rich unit and different functional groups carboxymethyl, hydroxymethyl, formyl,pyridylethenyl at the other extreme.Different routes and reaction conditions were explored to form these compounds, such as oxidation, reduction, cross-coupling, and Wittig-type reaction.All donors synthesized during the course of this work have been characterized by cyclic voltammetry and their oxidation potentials were determined by cyclic voltammetry. Molecular orbital diagram of the HOMO and LUMO of these new donors has been calculated with density functional theory (DFT). Charge transfer complexes of the donors with TCNQF4 were prepared and the electrical conductivity of these materials was measured, all CTCs are semi-conductive.In order to confirm such an assessment other experiments under different conditions (current intensity, solvent, donor concentration etc.) are in progress to obtain single crystals of such solids and then to correlate their electrical properties with their crystal structure.
Acknowledgments
This work received funding from the Directorate General for Scientific Research and Technological Development, DGRSDT.
References
- Becher, J.;Svenstrup, N. Synthesis 1995, 3, 215-235.
- Cava, M.P.;Pollack, N.M.;Dieterle, G.A. J. Am. Chem. Soc., 1973, 95, 2558-2560.
- Wudl, F.;Smith, G.M.;Hufnagel, E.J. J. Chem. Soc. Chem. Comm., 1970, 1453-1456.
- Bryce, M. R. Chem. Soc. Rev., 1991, 20, 355-390.
- Kraus, C. A. J. Am. Chem. Soc.,1913, 34, 1732-1741.
- S. Ameen, M. S. Akhtar, H. Shik Shin. Orient. J. Chem., 2013, 29, 837-860.
- McCoy, H. N.;Moore, W. C.J. Am. Chem. Soc.,1911, 33, 273-292.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2010.
- Simonsen, K.B.; Svenstrup, N.; Lau, J.; Simonsen, Ole.; Mork, P.; Kristensen, G.J.; Becher,J.Synthesis1996, 3, 407-418.
- Kimura, S.;Kurai, H.;Mori, T. Tetrahedron2002, 58, 1119-1124.
- Terauchi, T.;Sumi, S.;Kobayashi, Y.;Matsushita, Y.;Sato, A. Cryst. Growth Des.,2014,14, 1412-1418.
- Iyoda, M.; Kuwatani, Y.; Ueno, N.; Oda, M.J. Chem. Soc., Chem. Commun.,1992, 158-159.
- Gomper, R.; Hock, J.Synth. Met., 1997, 84, 339-340.
- Ashizawa, M.;Kimura, S.;Mori, T.;Misaki, Y.;Tanaka, K. Synthetic Metals2004,141, 307-313.
- M. Rahbar, A. Morsali, M. R. Bozorgmehr, S. A. Beyramabadi. Orient. J. Chem., 2015, 31, 1403-1407.
- M. Chandra Sekhar, A. Venkatesulu, T.Madhu Mohan, M. Gowrisankar. Orient. J. Chem., 2015, 31, 897-906.
- Segura, J.L.; Martin, N.Angew. Chem. Int. Ed., 2001, 40, 1372-1409.
- Fabre,J.M.J. Solid State Chem., 2002, 168, 367-383.
- Frère, P.;Skabara, P.J.Chem. Soc. Rev., 2005, 34, 69-68.

This work is licensed under a Creative Commons Attribution 4.0 International License.









