Synthesis and Characterization of Poly(α-Methylstyrene) by Cationic Polymerization Using a New Solid Ecological Catalyst
Moulkheir Ayat, Naima Bensaada*, Mohammed Belbachir, Amine Harrane, Rachid Meghabar
Polymer Chemistry Laboratory, Department of Chemistry, Faculty of Exact and Applied Sciences, University of Oran1 Ahmed Benbella, 31100 Oran, Algeria. Corresponding Author Email bensaadanaima@yahoo.fr
DOI : http://dx.doi.org/10.13005/ojc/310432
Article Received on :
Article Accepted on :
Article Published : 20 Oct 2015
The cationic polymerization of α-methylstyrene (AMS) is examined at 0°C in bulk and in solution in heterogenous phase using Maghnite-Na as a new solid ecological and efficient catalyst. Maghnite-Na is Algerian Montmorillonite sheet silicate clay, exchanged with sodium. Poly (α-methylstyrene) (PAMS) have been successfully prepared and characterized by differents techniques, such as, 1H NMR, 13C NMR, IR and DSC. The structural characteristics and thermal properties of the resulting polymers are elucidated. The influences of reaction temperature, initiator/monomer weight ratio and reaction time on the yields and the molecular weights are investigated. A mechanism for the reaction was proposed.
KEYWORDS:Solid catalysts; Montmorillonite; α-methylstyrene; Poly(α-methylstyrene);; Cationic polymerization; Mechanism
Download this article as:| Copy the following to cite this article: Ayat M, Bensaada N, Belbachir M, Harrane A, Meghabar R. Synthesis and Characterization of Poly(α-Methylstyrene) by Cationic Polymerization Using a New Solid Ecological Catalyst. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Ayat M, Bensaada N, Belbachir M, Harrane A, Meghabar R. Synthesis and Characterization of Poly(α-Methylstyrene) by Cationic Polymerization Using a New Solid Ecological Catalyst. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12016 |
Introduction
Cationic polymerization is a widely used method for preparing hydrocarbon polymers1. Numerous examples of the polymerization of vinyl monomers by a cationic pathway using various Lewis acids such as AlCl32 and BF33 catalyst systems can be found in the literature. These homogenous polymerization reactions are fast and efficient, using cheap catalysts, but molecular weight control is generally poor. Moreover, these homogeneous Lewis acid catalysts present some major drawbacks: their corrosive nature makes them difficult to handle and they are difficult to separate from the reaction products. Indeed the catalyst has to be removed from the polymer by a water-quenching step that not only destroys the Lewis acid, but also leads to a large volume of acidic aluminum waste4,5.
Recently, Hee-Jung and Dong-Ryul6 have prepared new nanocomposites from montmorillonite and some vinylic polymers such as PAMS. Another group developed the cationic polymerization of AMS initiated by different catalysts7,8. In others researches, Belbachir et al use a montmorillonite clay that exhibits good catalytic activity named “Maghnite” to induce the cationic polymerization of many heterocyclic and vinylic monomers9-13.
In continuation of studies on environmentally benign methods using solid supports, we use a new solid catalyst named Maghnite-Na in the cationic polymerization of AMS, which is the purpose of this article. Maghnite-Na is a new ecological solid catalyst; it does not imply the disposal of solvents or metal catalysts. This catalyst can be easily separated from the polymer product and regenerated by heating to a temperature above 100°C9. The effect of some factors such as the amount of the Maghnite-Na, the effect of the time on the polymerization and the mechanism of initiation are discussed.
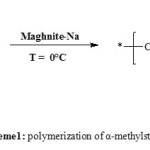 |
Scheme 1: polymerization of α-methylstyrene Click here to View scheme |
Materials and Methods
Reagents
The monomer AMS (99%; Aldrich, Paris, France) was purified by fractional distillation under reduced pressure. Methanol was dried over magnesium sulfate. Dichloromethane was used as received. Raw-Maghnite is Algerian Montmorillonite clay, was procured from ″BENTAL″ (Algerian Society of Bentonite).
Preparation of Maghnite-Na
The Clay is purified by separation of the argillaceous phase and the coarse phases. Rough clay is put in suspension in distilled water. In the suspension, the solide/liquide report/ratio is approximately 1/10. The suspension is then filtered on a sieve 0.02 mm in diameter of pores to eliminate the coarse matter and stones. It then versed in test-tubes and is left at rest during 2 hours. The separation of the argillaceous phase of the coarse fraction which remains at the bottom makes by siphonage. The recovered suspension is then centrifuged with 4500 tr/min during 20 min. Recovered clay is treated with a solution 1M of sodium Hexametaphosphate (NaPO3)6 (clay 20g in 100ml), by maintaining agitation, during 3h. The suspension is versed then in the test-tubes of separation and Na-Montmorillonite separate while exploiting its falling speed, MMT crosses with 20°C, a distance of 10 cm each 8h. Therefore Na-MMT is recovered by siphonage at a distance of 20 cm after 16h of decantation. One adds water distilled to the test-tubes after each siphonage, one agitates during 15min and one lets the suspension be elutriated before proceeding to new a siphonage. Na-MMT is then recovered by centrifugation with 4500 tr/min during 1h. With the end, it is washed with distilled water (on several occasions), filtered using one sintered of porosity 3 (maximum diameter of pores from 16 to 40 μm), dried in the drying oven with 105 °C, crushed using a mortar and kept in a desiccator14,15. In further work, we use the word Maghnite-Na in place of Na–MMT because it is original Maghnia.
Polymerization Procedures
The polymerization of AMS was carried out in a heterogeneous system. Each mixture was prepared with 16.9 mmol of AMS and 0.3g of Maghnite-Na and was introduced in several sealed tubes. The mixture was stirred with a magnetic stirrer. After a definite period of time, the resulting polymer was extracted with dichloromethane, precipitated in methanol, washed for several times, dried at 40°C in vacuum, and weighed. The monomer yield was determined gravimetrically by weighing the precipitated PAMS chains. The polymers were re-dissolved in dichloromethane and precipitated into methanol before characterization.
Polymer Characterization
Fourier Transform Infrared (FT-IR) spectroscopy (ATI Matson FTT N◦ 9501165) was used to confirm the structure of PAMS.
1H and 13C nuclear magnetic resonance (NMR) measurements were carried out on a 300 MHz Bruker spectrometer equipped with a probe BB05 mm, in CDCl3 solution under ambient temperature using Tetramethylsilane (TMS) as internal standard in these cases.
Gel-permeation chromatography was performed with a Spectra-Physics chromatograph, equipped with four columns connected in series, and packed with Ultrastyragel 103, 104, 105, 106 A˚ THF was used as Solvent and the instrument was calibrated to a first approximation with polystyrene of known molecular weights.
Differential scanning calorimetry (DSC) studies were conducted with a SETARAM-DSC92 in a nitrogen atmosphere at a heating rate of 10 °C/min.
Catalyst Characterization
X-ray diffraction (XRD) analysis of Maghnite-Na were obtained by using a Philips PW 1880 powder diffractometer using Cu-K α radiation (λ =1.541 Å).
Results and Discussion
Catalyst
Belbachir et al show that the Maghnite is a montmorillonite sheet silicate clay21, and it has 11.9 % more SiO2 than that from Wyoming 19.35 than from Montmorillon (Vienne, French). When treated with sulfuric acid, this difference is even greater; 14.21 % and 21.66 % as compared to Wyoming and Vienne Bentonite, respectively. Maghnite contains 5.60 % and 5.49 % less Al2O3, than the Wyoming and Vienne Bentonites, respectively 16,17.
The x-ray powder diffraction profiles shown in Figures 1 and 2 exhibited the presence of other crystalline phases such as quartz, feldspath and calcite in raw-Maghnite. Under acid treatment, all trace of calcite was removed in “Maghnite-H”. The increase in basal spacing from 12.5 Å in “raw- Maghnite”, characteristic of a single water layer between the sheets, to a 15.02 Å value in “Maghnite” for two interlamellar water layers reflects the changes in interlayer cation and its associated hydration state as a result of the acid treatment.
Others researchers reported that the decrease in the basal spacing indicates a loss of the interlayer water upon the replacement of Na+ for H+ 18,19.
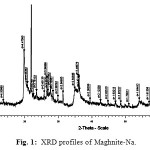 |
Figure 1: XRD profiles of Maghnite-Na. |
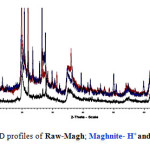 |
Figure 2: XRD profiles of Raw-Magh; Maghnite- H+and Maghnite-Na. Click here to View figure |
Sodium hexametaphosphate treatment of ‘‘r
Polymer
The structure of the PAMS was confirmed by IR, 1H NMR, 13C NMR and GPC measurements.
IR measurements
The IR spectrum of the polymer (Figure 3) showed principal bands characteristic of poly AMS: Peaks at 657,30 and 697,92 and 758,88cm-1 (out of plane C-H bending vibration), at 1029,23 and 1084,59cm-1 (C-C aromatic and aliphatic), at 3000cm-1 (C-H aromatic stretching).aw-maghnite’’ causes reduction in octahedral content (Al2O3) which resulted in an increase in the proportion of silica9. Maghnite-Na has a complete saturation of montmorillonite with sodium cations without destruction of catalyst structure. Similar results were obtained in previous works20-22.
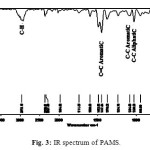 |
Figure 3: IR spectrum of PAMS. |
1H and 13C NMR Measurements
The 1H-NMR and 13C-NMR spectrum of the PAMS are shown in figures 4 and 5. The results are presented in Table 1 and 2, respectively. The cationic polymerization of AMS was examined in the presence of Maghnite-Na as a catalyst at 0°C for 6h.
The spectrum (Figure 4) showed different peaks, the methylene groups of the main chain as large signal in the range of 1.5–2 ppm, the terminal methyl groups at 1.2 ppm. Besides these well-known resonances, analysis shows at 5 ppm the characteristic resonance of the protons borne by the terminal double bond. Similar results are obtained using a Maghnite -H+ as catalyst14.
Table 1: 1H NMR Chemical shift for various protons of PAMS
|
Chemical shift δ( ppm ) |
6,720-7,193 |
5,100 |
1,838-1,879 |
1,671-1,725 |
1,564-1,621 |
1,033-1,053 |
|
Attribution |
2H2+2H3+1H4 |
1H7 |
2H6 |
3H5 |
3H8 |
3H1 |
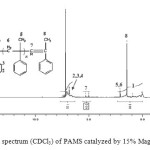 |
Figure 4: 1H NMR spectrum (CDCl3) of PAMS catalyzed by 15% Maghnite-Na,T =0°C. Click here to View figure |
Table 2: 13C-NMR Chemical shift for various carbons of PAMS
|
Chemical shift δ( ppm ) |
23,86
|
28,15
|
34,17
|
38,44
|
43,07
|
61,08
|
123,09 – 128,08
|
150,08 |
|
Attribution |
C-(8)
|
C-(1)
|
C-(6)
|
C (1’)
|
C (10)
|
C-(5)
|
C-(2,3,4,7,9)
|
C (4’) |
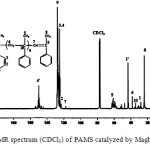 |
Figure 5: 13C NMR spectrum (CDCl3) of PAMS catalyzed by Maghnite-Na, T =0°C Click here to View figure |
DSC Measurements
Figure 6 shows the results of the DSC measurements. Glass transition temperature Tg of the resulting polymer was observed in the temperature range of 150-175°C. This analysis shows also closely neighbouring melting endotherms at temperatures between 225-250°C, This analysis indicates the semi-crystalline state of the resulted polymer.
 |
Figure 6: DSC measurements of PAMS Click here to View figure |
Effect of the amount of Maghnite-Na on the polymerization
The results of experiments of AMS polymerization induced by Maghnite-Na are reported in Table 3 which shows the effect of the amount of Maghnite-Na on the polymerization rate of AMS. Indeed, using various amounts of Maghnite-Na: 5; 7.5; 10; 15 and 20% by weight, the polymerization of AMS was carried in bulk at 0°C. For all these experiments the temperature was kept constant at 0°C for 6 hours. The amount of catalyst (Maghnite-Na /AMS) was an important factor of polymerization. We can see from Table 3 that the polymerization rate increased with the amount of Maghnite-Na, in which the effect of Maghnite-Na as a cationic catalyst for AMS polymerization is clearly shown. This phenomenon is probably the result of the number of ″initiating active sites″ responsible of inducing polymerization; this number is prorating to the catalyst amount used in reaction. Similar results were obtained in the polymerization of AMS14 by Maghnite-H+ and in the polymerization of styrene with Maghnite-Na22.
In contrast, as depicted in Table 3, the molecular weight is inversely proportional to the amount of Maghnite-Na. This finding is in good agreement with the proposal that Maghnite-Na is present as the active initiator species since the number of those species should be related to their surface area. Similar results are obtained by Chabani et al22.
Table 3: Polymerization of AMS induced by the Maghnite-Na
| Amount of Maghnite-Na (g) |
0.1 |
0.15 |
0.20 |
0.30 |
0.40 |
| Mn (g/mol) |
1440 |
1271 |
1222 |
1212 |
1015 |
| MW (g/mol) |
2084 |
2151 |
1722 |
1510 |
1732 |
| I = Mw/ Mn |
1.45 |
1.69 |
1.41 |
1.25 |
1.70 |
| Yield (%) |
49 |
55 |
65 |
69 |
75 |
Effect of the time on the polymerization
Figure 7 shows the yield of polymer versus time for the polymerization of AMS using Maghnite-Na as catalyst. As the figure shows that at the end of 4h, polymerization takes place quickly and reaching a best yield of 75% at the end of 6h in the presence of 15% of Maghnite-Na at 0°C, after this time the polymerization slows down gradually and the yield becomes almost constant.
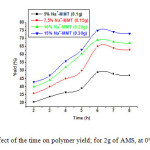 |
Figure 7: Effect of the time on polymer yield; for 2g of AMS, at 0°C in bulk. |
Mechanism of polymerization
Maghnite-Na is a cation -exchanged montmorillonite sheet silicate clay. The Montmorillonite lattice is composed of layers made up of two silica tetrahedral sheets with a central alumina octahedral sheet. AMS polymerizes cationically by opening of the double bond in the monomer. According to the foregoing discussion and the results of product analysis, we may suggest a cationic mechanism for the resulting reaction of polymerization induced by Maghnite-Na. Cations carried by Montmorillonite sheets of Maghnite-Na (Scheme 2) induced the cationic polymerization Propagation and termination then take place by conventional cationic mechanism (Scheme 3). Termination occurs by proton transfer to monomer and/or to initiator produced by unsaturation as shown in the reaction (Schemes 4).
Initiation
Initiation is done between the initiator and a first molecule of the monomer to form the active species (Scheme2).
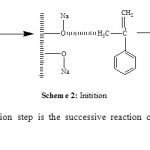 |
Scheme 2 Click here to View scheme |
Propagation
The propagation step is the successive reaction of AMS with the carbocation intermediate (Scheme 3).
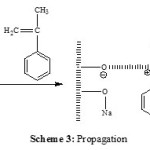 |
Scheme 3 Click here to View scheme |
Termination
We suppose that there was formation of a double bond at the end of the chain of the AMS by spontaneous transfer (Scheme 4-1) or by methanol added as quenching agent to the PAMS growing chains (Scheme 4-2) and regeneration of catalyst.
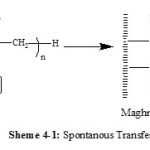 |
Scheme 4 Click here to View scheme |
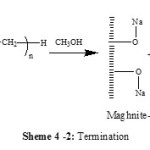 |
Scheme 4 B Click here to View scheme |
Conclusions
The present work shows that AMS polymerization can be induced in heterogeneous phase by sodium exchanged montmorillonite clay called Maghnite-Na. PAMS was successfully obtained by direct method by the use of a cheap non-toxic catalyst and by an easy-to-handle procedure and just by filtering, the catalyst can be separated from the reaction mixtures. The polymerization was found to be initiated by Maghnite-Na powder. The structure of the polymer is confirmed with 1H and 13C NMR spectra. The mechanism of this reaction involves both, Brønsted and Lewis acidic sites on the clay interface, to initiate the polymerization. This chemistry may be a potential direct method to produce PAMS/Montmorillonite nanocomposites.
References
- Sigwalt, P. Recent Results in Proton-Initiated and Non Proton-Initiated Cationic Polymerizations. Polym. J. 1985, 17, 57-71.
- Ribeiro, R.M.; Portela, M.F.; Deffieux, A.; Cramail, H.; Rocha, M. Isoespecific homo and copolymerization of styrene with ethylene in the presence of VCl3, AlCl3 as catalyst. Macromol. Rapid. Commun. 1996, 17, 461-469.
- Satoh, K.; Nakashima, J.; Kamigaito, I.; Sawamoto, M. Novel BF3OEt2/R-OH initiating system for controlled cationic polymerization of styrene in the presence of water. Macromolecules. 2001, 34, 396-401.
- Clark, J.H.; Monks, G.L.; Nightingale, D.J.; Price, P.M.; White, J.F. A new solid acid-based route to linear alkybenzenesy. J. Catal. 2000, 193, 348-350.
- Köppl, A.; Alt, H.G.; Phillips, M.D.: Partially hydrolyzed trimethylaluminum (PHT) as heterogeneous cocatalyst for the polymerization of olefins with metallocene complexes. J. Appl. Polym. Sci. 2001, 80, 454-466.
- Jung, H.K.; Dong, R. K.; U. S. Patent 2006, 7, 115, 683.
- Banerjee, S.; Paira, T.K.; Mandal, T.K. Control of molecular weight and tacticity in stereospecific living cationic polymerization of α-methylstyrene at 0°C using FeCl3-based initiators: Effect of tacticity on thermal properties. Macromol. Chem. Phys. 2013, 214, 1332-1344.
- Li, D.; Hadjikyriacous, S.; Faust, R. Living carbocationic polymerization of α-Methylstyrene using tin halides as coinitiators. Macromolecules. 1996, 29, 6061-6067.
- Belbachir, M.; Bensaoula, A. Composition and method for using bentonites. US. Patent 2006, 7, 094, 823 B2.
- Belbachir, M.; Harrane, A.; Megharbi, R. Maghnite, a green catalyst for cationic polymerization of vinylic and cyclic monomers. Macromol. Symp. 2006, 1-4, 245-246.
- Harrane, A.; Naar, N.; Belbachir, M. Ring opening polymerization of oxetane by the use of a montmorillonite clay as catalyst. Mater. Lett. 2007, 61, 3555-3558.
- Bensaada, N.; Meghabar, R.; Belbachir, M. The Synthesis and Characterization of Linear Poly (divinylbenzene-co-ethylvinylbenzene) Via A Cationic Solid Catalyst. Orient. J. Chem. 2013, 29, 1631-1635.
- Bouhadjar, L. ; Hachemaoui, A.; Yahiaoui, A.; Bouchama A.; Chikh, K.; Belbachir, M. Synthesis of Poly[(pyrrole-2,5-diyl)-co-(4-hydroxylbenzylidene)] Catalysed byMaghnite– H+. Orient. J. Chem. 2013, 29, 1615-1620.
- Ayat, M.; Harrane, A.; Belbachir, M. Maghnite‐H+ a solid catalyst for the cationic polymerization of α‐methylstyrene. J. Appl. polym. Sci. 2008, 109, 1476-1479.
- Haous, M.; Harrane, A.; Belbachir, M.; Taulelle, F. Solid State NMR Characterization of formation of Poly (Ԑ-caprolactone) / Maghnite Nanocomposites by In‐situ Polymerization. J. Polym. Sci. Part B: Polym. Phys. 2007, 45, 3060-3068.
- Kerr, P.F.; Hamilton, P.K.; Pill, R.J. Analytical data on reference, Clay Minerals: American Petroleum Institute Project 49. Clay Minerals Standards, preliminary report, 1950, 7, 92-98.
- Damour, A.; Salvetat, D. Analyses sur un hydrosilicate d’alumine trouvé à Montmorillon. Ann. Chim. Phys. 1993, 3, 376–383.
- Jianzhong, M.; Dangge, G.; Xinjiang, C.; Yun, C. Synthesis and Characterization of VP/O-MMT Macromolecule Nanocomposite Material via Polymer Exfoliation–Adsorption Method. J. Compos. Mater. 2006, 40, 2279-2286.
- Kwon, O.Y.; Park, K.W.; Jeong, S. Preparation of Porous Silica-Pillared Montmorillonite: Simultaneous Intercalation of Amine-Tetraethylorthosilicate into H-Montmorillonite and Intra-Gallery Amine-Catalyzed Hydrolysis of Tetraethylorthosilicate. Bull. Korean Chem. Soc. 2001, 22, 678-686.
- Breen, C.; Madejová, J.; Komadel, P. Characterization of moderately acid-treated, size-fractionated montmorillonites using IR and MAS NMR spectroscopy and thermal analysis. J. Mater. Chem. 1995, 5, 469-474.
- Chabani, M.; Yahiaoui, A.; Hachemaoui, A.; Belbachir, M. New Approach for the Polymerization of 2-Chloroethyl Vinyl Ether Using a Maghnite Clay as Eco-Catalyst. J. Appl. polym. Sci. 2011, 122, 1800-1806.
- Bensaada, N.; Ayat, M.; Meghabar, R.; Belbachir, M. The synthesis of polystyrene with a new chemical approach. Current Chemistry Letters 2015, 4, 55-60.

This work is licensed under a Creative Commons Attribution 4.0 International License.









