Synthesis and anti-inflammatory activity of some new thiadiazole linked pyrazole benzene sulphonamides as cyclooxygenase inhibitors
Jahangir Alam, Ozair Alam*, Rahmat Ali, Mohd. Javed Naim, Suroor Ahmad Khan
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Jamia Hamdard, New Delhi-110062, India
Corresponding Authors Email: dr.ozairalam@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310404
Article Received on :
Article Accepted on :
Article Published : 05 Dec 2015
A new series of thiadiazole linked pyrazole benzenesulfonamide derivatives were synthesized by the condensation of aldehydic pyrazole with aryl substituted thiadiazole amine followed by Schiff base reaction. The synthesized compounds (6a-o) were characterized by IR, NMR, and Mass spectral data, further evaluated their in-vivo anti-inflammatory, analgesic and in-vitro COX-II inhibition assay. The compounds 6b and 6m showed most significant in-vivo anti-inflammatory with 72.33 and 71.17% inhibition along analgesic activity having 67.89% and 71.37 % respectively. Their selectivity against COX-II enzyme with selectivity index 67.81 and 66.38 was established for 6b and 6m, which is compared with Celecoxib. During the gastric ulceration study, selected compounds couldn’t observed any ulcerogenic effect on gastric mucosa.The in-silico pharmacokinetic profile and molecular docking study exposed very good binding affinity towards the cycloxygenase (COX-II) enzyme (PDB Id: 3PGH), therefore the compounds 6b and 6m are used as promising lead candidates for the support of drug development.
KEYWORDS:Benzenesulfonamide; Cyclooxygenase; Prostanoids; Traumatic infections
Download this article as:| Copy the following to cite this article: Alam J, Alam O, Ali R, Naim M. J, Khan S. A. Synthesis and anti-inflammatory activity of some new thiadiazole linked pyrazole benzene sulphonamides as cyclooxygenase inhibitors. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Alam J, Alam O, Ali R, Naim M. J, Khan S. A. Synthesis and anti-inflammatory activity of some new thiadiazole linked pyrazole benzene sulphonamides as cyclooxygenase inhibitors. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=13037 |
Introduction
Tissues in response to traumatic infections, post-ischaemia, toxicity and autoimmune injury cause inflammation and pain. The pathophysiological conditions such as evolution of persistent tissue damage of leucocytes, lymphocytes and collagen, the defensive process normally lead to recovery from noxious stimulus1. According to WHO report approximately 90% of the illness are associated with inflammation and pain2. The inflammatory mediators of eicosanoids are activated by the nociceptors which leads to hyperalgesia3. The key focus of medicine is to reduce the pain and classical inflammation such as fever, redness and swelling, which are mediated by pro-inflammatory eicosanoids4. During eicosanoids pathway the cellular enzymatic activity of arachidonic acid produces the prostanoids(a pain inducer) which is initiated by cyclooxygenase5. Cyclooxygenase (EC 1.14.99.1) enzyme is an inter-convertible form of Cyclooxygenases I and II (COX-I, COX-II), both of this enzyme hassimilar molecular weight, approximately 70 and 72 kDa.TheCOX-I is responsible in physiological function and COX-IIis responsible for inflammation and pain6. For the treatment of pain and inflammation in rheumatoid arthritis and inflammatory diseases have been the most widely used are non-steroidal anti-inflammatory drugs (NSAIDs)7.The conventional and most frequent use of NSAIDs causes severe side effects such as irritation of the gastric mucosa and damage to gastrointestinal tract. This new era of research, our main aim is to investigate a newer and safe anti-inflammatory agents. The COX-II inhibitors are a main target for management of pain and inflammation8.Some of the marketed drug such as celecoxib and etoricoxib are very selective COX-II inhibitors which showed little gastric irritation and also decreases the risk of peptic ulceration. But still some of the adverse effects of selective COX-II inhibitors are observed during clinical study9. Hence our aim is to find a new safer and potent compound having as analgesic and anti-inflammatory as selective COX-II inhibitors along with less gastric toxicities.
The thiadiazole and pyrazole derivatives represent a class of organic compounds of great importance in biological chemistry. For instance, thiadiazole and pyrazole derivatives shows anticancer10, antibacterial11, antiviral12, antitubercular13, antifungal14, anti-inflammatory15, antiprotozoal16, Cardioprotective17, antidepressant properties18, analgesic activities19.Searching these compounds we have found thiadiazole linked pyrazole benzene sulphonamides are one of the moieties on which studied have been focused (Figure-1). In our laboratory, we have designed (Figure-2) and synthesized some 4-(5-chloro-3-methyl-4-(((5-phenyl-1,3,4-thiadiazol-2-yl)imino)methyl)-1H-pyrazol-1-yl)benzenesulfonamide derivatives (6a-o)in search for new compound with expected biological activities. We hereby report the thiadiazole linked pyrazole benzene sulphonamides and their characterization by IR, NMR and Mass spectrometry techniques. Newly synthesized compounds were also screened for their anti-inflammatory, Analgesic, Ulcerogenic and in-vitro COX-II inhibitory assay.
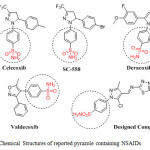 |
Figure 1: Chemical Structures of reported pyrazole containing NSAIDs. Click here to View figure |
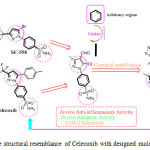 |
Figure 2: The structural resemblance of Celecoxib with designed molecule is shown. Click here to View figure |
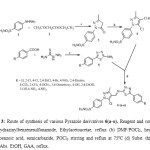 |
Figure 3: Route of synthesis of various Pyrazole derivatives 6(a-o). Reagent and conditions: (a) 4-hydrazinylbenzenesulfonamide, Ethylacetoacetae, reflux (b) DMF/POCl3, heating (c) Subst. benzoic acid, semicarbazide, POCl3 stirring and reflux at 750C (d) Subst. thiadiazole amine, Abs. EtOH, GAA, reflux. Click here to View figure |
Materials and Method
Chemistry
Melting points were determined by the open capillary method with electrical melting point apparatus and are uncorrected. IR spectra were recorded as KBr (pellet) on Bio Rad FT-IR spectrophotometer and 1H-NMR and 13C-NMR spectra were recorded on Bruker DPX 300 MHz spectrophotometer using DMSO-d6 or CDCl3 as NMR solvent. Mass spectra were recorded on JEOL SX102/DA-6000 mass spectrometer using m-nitrobenzylalcohol as a matrix and elemental analysis on Vario-EL III CHNOS-Elemental analyzer. Thin Layer Chromatography (TLC) was performed to monitor progress of the reaction and purity of the compounds, the spot being located under iodine vapours or UV-light.
Synthesis of 4-(3-methyl-5-oxo-4,5-dihydro-1H-pyrazol-1-yl)benzenesulfonamide (3)
3-methyl-1-phenyl-1H-pyrazol-5(4H)-one (3) was synthesized by heating equimolar mixture of 4-hydrazinylbenzenesulfonamide (1) and ethyl acetoacetate (2) at 110-120 °C for 4 h. The reaction mixture was cooled and ether (20 ml) is added to it. The mixture was stirred to give a solid product. It was filtered, washed with ether and recrystallized from ethanol to give the desired product as a pale yellow crystalline compound20. Yield 75%, mp 122-124 °C
Synthesis of 4-(5-chloro-4-formyl-3-methyl-1H-pyrazol-1-yl)benzenesulfonamide (4)
Compound (3) in100mlround bottom flask were dissolved in dry DMFand it was cooled to 0°C and treated dropwise with phosphorous oxychloride (POCl3), maintaining the temperature between 10-15 °C. The reaction mixture was heated on a steam bath for 1 h, cooled and poured into crushed ice with stirring. The separated product was filtered and washed with water to obtained 4-(5-chloro-4-formyl-3-methyl-1H-pyrazol-1-yl) benzenesulfonamide(4). It was recrystallized from ethanol and obtained as yellow needles20. Yield 74%, mp 147°C;IR (KBr) cm-1: 3167 (SO2NH2), 3079(C-H of CHO), 1667 (C=O), 1595 (C=N), 1327, 1159 (SO2), 1012 (C-N).1H-NMR (400 MHz, CDCl3): δ 2.53 (s, 3H, Me),6.73 (bs, 2H, SO2NH2 , D2O exchangeable), 7.53 (d, 2H, Ph H-2,6, J=7.4 Hz), 7.51 (d, 2H, Ph H-3,5, J=7.8), 10.32 (s, 1H, CHO);13C-NMR (125 MHz, CDCl3): δ183.81 (CHO), 151.7 (C-3), 136.9 (Ph C-1), 133.4 (C-5), 129.2 (Ph C-3,5), 129.1 (Ph C-4), 125.1 (Ph C- 2,6), 117.3 (C-4,CHO), 13.8 (Me);ESI-MS: m/z 301.08, (M+2).Anal. calcd for C11H10ClN3O3S :C, 44.08; H, 3.36; N, 14.04;Found: C, 44.25; H, 3.26; N, 14.78;%.
Substituted-5-phenyl-1, 3, 4-thiadiazol-2-amine (5)
Substituted-5-phenyl-1, 3, 4-thiadiazol-2-amine (5) was synthesized by according to reported literature21.Equimolar mixture of substituted benzoic acid and semicarbazide in 100ml round bottom flask, POCl3 (13 ml) were added to it and heated at 75 0Cfor half an hour. After cooling down to room temperature then add water. The reaction mixture was reflux for 4 hr, after cooling the mixture was basified to PH-8 by drop wise addition of 50 % NaOH solution under the stirring. The precipitate was filtered and recrystallized from ethanol to obtained pure yield of compounds. 5-phenyl-1,3,4-thiadiazol-2-amine(5a);Yield: 61%; m.p.: 222-224°C; IR (KBr) cm-1: 3283, 3117 (NH2), 1642 (C=N), 696 (C=S); 1H-NMR (300 MHz, DMSO-d6); δ 7.42 (s, 2H), 7.47-7.52 (dd, J= 6.4 Hz, J=14.00 Hz, 2H), 7.83 (d, 2H), ESI-MS: m/z [M+H] 178.31; Anal. calcd for C8H7N3S: C, 54.22; H, 3.98; N, 23.71; Found: C, 54.25; H, 3.97; N, 23.73; %.
Synthesis of (E)-4-(5-chloro-3-methyl-4-(((5-substituted-phenyl-1,3,4-thiadiazol-2-yl)methylene)amino)-1H-pyrazol-1-yl)benzenesulfonamide derivatives (6a-6o)
In a 100ml of round-bottom flask, a equimolar mixture compound (4)were dissolve in 30 ml of absolute ethanol, catalytic amount of Glacial acidic acid (0.3ml) added to it. Equimolar amount of compound (5) was added and the mixture was refluxed for 6 hr. The reaction was monitored by TLC until the disappearance of starting materials, precipitates come out and filtered, washed with Ethanol, dried and recrystallized from ethanol to obtained white solid product. Progress of the reaction was checked by TLC using ethyl acetate: hexane (8:2) as solvent system.
(E)-4-(5-chloro-3-methyl-4-(((5-phenyl-1,3,4-thiadiazol-2-yl)methylene)amino)-1H-pyrazol-1-yl)benzenesulfonamide (6a)
Yield: 58%; m.p.: 174-176°C; IR (KBr) cm-1: 3373(SO2NH2), 1634 (C=N), 1550 (C=C), 1343(SO2NH2), 1012 (C-N).1H-NMR (300 MHz, DMSO-d6); δ 2.39 (s, 3H, Pyrazole-CH3), 3.43 (bs, 2H, SO2NH2 , D2O exchangeable),4.61 (bs, H, HC=N), 7.33 (s, 1H, Ar-H), 7.57(d, 2H, Ar-H J = 7.5 Hz), 7.77 (d, 2H, Ar-H J = 7.1 Hz),7.93 (d, 2H, Ar-H J = 7.6 Hz), 8.11 (d, 2H, Ar-H J = 7.8 Hz).13C-NMR (75 MHz, DMSO-d6); δ 14.39, 114.23, 123.27, 128.11, 130.16, 135.72, 141.51, 150.09(C-Pyrazolo), 161.91(HC=N), 173.34(C-thiadiazole). ESI-MS: m/z 460.13, (M+2).Anal. calcd for C19H15ClN6O2S2:C, 49.72; H, 3.29; N, 18.31; Found: C, 49.77; H, 3.28; N, 18.32; %.
(E)-4-(5-chloro-4-(((5-(2-chlorophenyl)-1,3,4-thiadiazol-2-yl)methylene)amino)-3-methyl-1H-pyrazol-1-yl)benzenesulfonamide (6b)
Yield: 63%; m.p.: 176-178°C; IR (KBr) cm–1: 3376(SO2NH2), 3154 (N-H), 1638 (C=N), 1555 (C=C), 1346(SO2NH2) 1014 (C-N), 754(C-Cl).1H-NMR (300 MHz, DMSO-d6); δ 2.42 (s, 3H, Pyrazole-CH3), 3.45 (bs, 2H, SO2NH2 , D2O exchangeable), 4.65 (bs, H, HC=N), 7.33 (s, 1H, Ar-H), 7.57(d, 1H, Ar-H J = 7.5 Hz), 7.77 (d, 2H, Ar-H J = 7.1 Hz),7.96 (d, 2H, Ar-H J = 7.6 Hz), 8.03 (d, 2H, Ar-H J = 7.8 Hz).13C-NMR (75 MHz, DMSO-d6); δ 14.41, 114.25, 123.29, 128.08, 130.18, 135.71, 141.53, 150.12(C-Pyrazolo), 161.94(HC=N), 173.30(C-thiadiazole). ESI-MS: m/z 494.13, (M+2).Anal. calcd forC19H14Cl2N6O2S2: C, 46.25; H, 2.86; N, 17.03; Found: C, 46.39; H, 2.78; N, 17.11; %.
(E)-4-(5-chloro-4-(((5-(4-chlorophenyl)-1,3,4-thiadiazol-2-yl)imino)methyl)-3-methyl-1H-pyrazol-1-yl)benzenesulfonamide (6c)
Yield: 65%; m.p.: 167-169°C; IR (KBr) cm−1: 3375 (SO2NH2), 3152 (N-H), 1633 (C=N), 1556 (C=C), 1344 (SO2NH2), 1010 (C-N), 750 (C-Cl).1H-NMR (300 MHz, DMSO-d6); δ 2.41 (s, 3H, Pyrazole-CH3), 3.48 (bs, 2H, SO2NH2 , D2O exchangeable), 4.63 (bs, H, HC=N), 7.54 (d, 2H, Ar-H, J = 7.3 Hz), 7.79 (d, 2H, Ar-H J = 7.2 Hz), 7.90 (d, 2H, Ar-H J = 7.6 Hz), 8.03 (d, 2H, Ar-H J = 7.8 Hz). 13C-NMR (75 MHz, DMSO-d6); δ 14.43, 114.24, 123.32, 128.07, 130.17, 135.74, 141.51, 150.14 (C-Pyrazolo), 161.93 (HC=N), 173.31(C-thiadiazole). ESI-MS: m/z 494.19, (M+2). Anal. calcd for C19H14Cl2N6O2S2: C, 46.25; H, 2.86; N, 17.03; Found: C, 46.37; H, 2.77 ; N, 17.12; %.
(E)-4-(5-chloro-4-(((5-(2,4-dichlorophenyl)-1,3,4-thiadiazol-2-yl)imino)methyl)-3-methyl-1H-pyrazol-1-yl)benzenesulfonamide (6d)
Yield: 68%; m.p.: 174-176°C; IR (KBr) cm−1: 3373 (SO2NH2), 3151 (N-H), 1628 (C=N), 1553 (C=C), 1343 (SO2NH2), 1015 (C-N), 767 (C-Cl). 1H-NMR (300 MHz, DMSO-d6); δ 2.48 (s, 3H, Pyrazole-CH3), 3.43 (bs, 2H, SO2NH2 , D2O exchangeable), 4.60 (bs, H, HC=N), 7.54 (d, H, Ar-H, J =6.8 Hz), 7.61 (d, H, Ar-H), 7.79 (d, H, Ar-H J = 7.2 Hz), 7.90 (d, 2H, Ar-H J = 7.6 Hz), 8.03 (d, 2H, Ar-H J = 7.8 Hz).13C-NMR (75 MHz, DMSO-d6); δ 14.42, 114.27, 123.30, 128.11, 130.14, 135.71, 141.49, 150.11 (C-Pyrazolo), 161.89 (HC=N), 173.32 (C-thiadiazole). ESI-MS: m/z 525.39, [M-2]. Anal. calcd for C19H13Cl3N6O2S2: C, 43.23; H, 2.48; N, 15.92; Found: C, 43.33; H, 2.43; N, 15.86; %.
(E)-4-(4-(((5-(4-bromophenyl)-1,3,4-thiadiazol-2-yl)imino)methyl)-5-chloro-3-methyl-1H-pyrazol-1-yl)benzenesulfonamide(6e)
Yield: 62%; m.p.: 178-179°C; IR (KBr) cm−1: 3374 (SO2NH2), 3147 (N-H), 1626 (C=N), 1547 (C=C), 1347 (SO2NH2), 1016 (C-N), 821 (C-Br).1H-NMR (300 MHz, DMSO-d6); δ 2.45 (s, 3H, Pyrazole-CH3), 3.43 (bs, 2H, SO2NH2 , D2O exchangeable), 4.67 (bs, H, HC=N), 7.54 (d, 2H, Ar-H, J = 7.3 Hz), 7.79 (d, 2H, Ar-H J = 7.2 Hz), 7.90 (d, 2H, Ar-H J = 7.6 Hz), 8.03 (d, 2H, Ar-H J = 7.8 Hz).13C-NMR (75 MHz, DMSO-d6); δ 14.45, 114.22, 123.29, 128.06, 130.12, 135.71, 141.49, 150.11 (C-Pyrazolo), 161.96 (HC=N), 173.37(C-thiadiazole). ESI-MS: m/z 537.71, (M+2). Anal. Calcd for C19H14BrClN6O2S2: C, 42.43; H, 2.62; N, 15.63; Found: C, 42.39; H, 2.67; N, 15.72; %.
(E)-4-(5-chloro-3-methyl-4-(((5-(4-nitrophenyl)-1,3,4-thiadiazol-2-yl)imino)methyl)-1H-pyrazol-1-yl)benzenesulfonamide (6f)
Yield: 74%; m.p.: 169-171°C; IR (KBr) cm−1: 3346 (SO2NH2), 33247 (N-H), 1632 (C=N), 1579 (C=C), 1538 (N=O), 1332 (SO2NH2), 1013 (C-N).1H-NMR (300 MHz, DMSO-d6); δ 2.44 (s, 3H, Pyrazole-CH3), 3.57 (bs, 2H, SO2NH2 , D2O exchangeable), 4.76 (bs, H, HC=N), 7.81 (d, 2H, Ar-H, J = 7.3 Hz), 7.93 (d, 2H, Ar-H J = 7.2 Hz), 8.07 (d, 2H, Ar-H J = 7.6 Hz), 8.30 (d, 2H, Ar-H J = 7.8 Hz).13C-NMR (75 MHz, DMSO-d6); δ 14.53, 114.21, 123.39, 128.05, 130.11, 135.61, 141.69, 150.09 (C-Pyrazolo), 161.89 (HC=N), 173.35 (C-thiadiazole). ESI-MS: m/z 503.49, (M+). Anal. Calcd for C19H14ClN7O2S2: C, 45.28; H, 2.80; N, 19.46; Found: C, 45.33; H, 2.75; N, 19.52; %.
(E)-4-(5-chloro-4-(2-(5-(2, 4-dinitrophenyl)-1, 3, 4-thiadiazol-2-yl) vinyl)-3-methyl-1H-pyrazol-1-yl) benzenesulfonamide (6g)
Yield: 73%; m. p.: 180-182°C; IR (KBr) cm−1: 3343 (SO2NH2), 3168 (N-H), 1633 (C=N), 1540 (C=C), 1542 (N=O), 1331 (SO2NH2), 1012 (C-N).1H-NMR (300 MHz, DMSO-d6); δ 2.48 (s, 3H, Pyrazole-CH3), 3.54 (bs, 2H, SO2NH2 , D2O exchangeable), 4.73 (bs, H, HC=N), 7.79 (d, 2H, Ar-H, J = 7.3 Hz), 7.87 (d, 2H, Ar-H J = 7.2 Hz), 8.31 (d, H, Ar-H), 8.69 (d, 2H, Ar-H);Anal. Calcd for C19H13ClN8O6S2: C, 41.57; H, 2.39; N, 20.41; Found: C, 41.48; H, 2.37; N, 20.49; %.
(E)-4-(5-chloro-3-methyl-4-(((5-(o-tolyl)-1,3,4-thiadiazol-2-yl)imino)methyl)-1H-pyrazol-1-yl)benzenesulfonamide (6h)
Yield: 70%; m.p.: 175-177°C; IR (KBr) cm−1: 3375 (SO2NH2), 3158 (N-H), 1632 (C=N), 1547 (C=C), 1347 (SO2NH2), 1013 (C-N). 1H-NMR (300 MHz, DMSO-d6); δ 2.58 (s, 3H, Phenyl-CH3), 2.69 (s, 3H, Pyrazole-CH3), 3.58 (bs, 2H, SO2NH2 , D2O exchangeable), 4.54 (bs, H, HC=N), 7.33 (s, 1H, Ar-H), 7.57(d, 2H, Ar-H J = 7.5 Hz), 7.77 (d, H, Ar-H J = 7.1 Hz),7.93 (d, 2H, Ar-H J = 7.6 Hz), 8.11 (d, 2H, Ar-H J = 7.8 Hz).13C-NMR (75 MHz, DMSO-d6); δ 13.39, 18.64, 114.43, 123.28, 128.12, 130.18, 135.70, 141.56, 150.08(C-Pyrazolo), 161.93(HC=N), 173.32 (C-thiadiazole). Anal. calcd for C20H17ClN6O2S2: C, 50.79; H, 3.62; N, 17.77; Found: C, 50.83; H, 3.57; N, 17.85; %
(E)-4-(5-chloro-3-methyl-4-(((5-(p-tolyl)-1,3,4-thiadiazol-2-yl)imino)methyl)-1H-pyrazol-1-yl)benzenesulfonamide (6i)
Yield: 76%; m. p.: 173-175°C; IR (KBr) cm−1: 3371(SO2NH2), 3169 (N-H), 1635 (C=N), 1551 (C=C), 1342 (SO2NH2), 1011 (C-N).1H-NMR (300 MHz, DMSO-d6); δ 2.35 (s, 3H, Phenyl-CH3), 2.46 (s, 3H, Pyrazole-CH3), 3.45 (bs, 2H, SO2NH2 , D2O exchangeable),4.60 (bs, H, HC=N), 7.33 (s, 2H, Ar-H), 7.57(d, 2H, Ar-H J = 7.5 Hz), 7.77 (d, 2H, Ar-H J = 7.1 Hz),7.93 (d, 2H, Ar-H J = 7.6 Hz).Anal. calcd for C20H17ClN6O2S2: C, 50.79; H, 3.62; N, 17.77; Found: C, 50.83; H, 3.57; N, 17.85; %.
(E)-4-(5-chloro-4-(((5-(4-methoxyphenyl)-1,3,4-thiadiazol-2-yl)imino)methyl)-3-methyl-1H-pyrazol-1-yl)benzenesulfonamide (6j):
Yield: 78%; m. p.: 167-169°C; IR (KBr) cm−1: 3368 (SO2NH2), 3180 (N-H), 1632 (C=N), 1530 (C=C), 1358 (SO2NH2), 1546 (C-O-C), 1016 (C-N). 1H-NMR (300 MHz, DMSO-d6); δ 2.44 (s, 3H, Pyrazole-CH3), 3.81 (s, 3H, Phenyl-OCH3), 3.38 (bs, 2H, SO2NH2 , D2O exchangeable), 4.52 (bs, H, HC=N), 7.33 (s, 2H, Ar-H), 7.57(d, 2H, Ar-H J = 7.5 Hz), 7.77 (d, 2H, Ar-H J = 7.1 Hz),7.93 (d, 2H, Ar-H J = 7.6 Hz).Anal. calcd for C20H17ClN6O3S2: C, 49.13; H, 3.50; N, 17.19; Found: C, 49.17; H, 3.43; N, 17.26 ; %.
(E)-4-(5-chloro-4-(((5-(3,4-dimethoxyphenyl)-1,3,4-thiadiazol-2-yl)methylene)amino)-3-methyl-1H-pyrazol-1-yl)benzenesulfonamide (6k):
Yield: 74%; m. p.: 182-184°C; IR (KBr) cm−1: 3378 (SO2NH2), 3184 (N-H), 1636 (C=N), 1534 (C=C), 1354 (SO2NH2), 1254 (C-O-C), 1015 (C-N).1H-NMR (300 MHz, DMSO-d6); δ 2.45 (s, 3H, Pyrazole-CH3), 3.73 (s, 6H, Phenyl- 2×OCH3), 3.65 (bs, 2H, SO2NH2 , D2O exchangeable), 4.57 (bs, H, HC=N), 7.33 (s, 2H, Ar-H), 7.57(d, 1H, Ar-H J = 7.5 Hz), 7.77 (d, 2H, Ar-H J = 7.1 Hz),7.93 (d, 2H, Ar-H J = 7.6 Hz).13C-NMR (75 MHz, DMSO-d6); δ 13.45, 56.87, 111.03, 112.71, 114.73, 120.60, 123.82, 126.16, 127.65, 133.61, 140.43, 149.12, 150.40, 160.04 (C=O), 174.81 (Aromatic NH-C=O); ESI-MS: m/z 504.13, [M+2]; Anal. calcd for C21H19ClN6O4S2: C, 48.60; H, 3.69; N, 16.19; Found: C, 48.73; H, 3.53; N, 16.26; %.
(E)-4-(5-chloro-4-(((5-(4-hydroxyphenyl)-1,3,4-thiadiazol-2-yl)imino)methyl)-3-methyl-1H-pyrazol-1-yl)benzenesulfonamide (6l):
Yield: 77%; m.p.: 169-171°C; IR (KBr) cm−1: 3373 (SO2NH2), 3179 (N-H), 3430(O-H), 1631 (C=N), 1538 (C=C), 1357 (SO2NH2), 1014 (C-N). 1H-NMR (300 MHz, DMSO-d6); δ 2.42 (s, 3H, Pyrazole-CH3), 3.55 (bs, 2H, SO2NH2 , D2O exchangeable), 4.68 (bs, H, HC=N), 5.39 (s,1H, OH), 7.57(d, 2H, Ar-H J = 7.5 Hz), 7.77 (d, 2H, Ar-H J = 7.1 Hz),7.93 (d, 2H, Ar-H J = 7.6 Hz), 8.11 (d, 2H, Ar-H J = 7.8 Hz).13C-NMR (75 MHz, DMSO-d6); δ 13.23, 114.12, 116.04, 123.27, 126.34, 128.82, 133.13, 140.32, 149.45, 158.03 (C-Pyrazolo), 160.23 (HC=N), 174.32 (C-thiadiazole). Anal. calcd for C19H15ClN6O3S2: C, 48.05; H, 3.18; N, 17.69; Found C, 48.24; H, 3.12; N, 17.72; % .
(E)-4-(5-chloro-4-(((5-(2,4-dihydroxyphenyl)-1,3,4-thiadiazol-2-yl)imino)methyl)-3-methyl-1H-pyrazol-1-yl)benzenesulfonamide (6m):
Yield: 73%; m.p.: 186-1188°C; IR (KBr) cm−1: 3372 (SO2NH2), 3174 (N-H), 3435(O-H), 1645 (C=N), 1533 (C=C), 1356 (SO2NH2), 1017 (C-N). 1H-NMR (300 MHz, DMSO-d6); δ 2.47 (s, 3H, Pyrazole-CH3), 3.53 (bs, 2H, SO2NH2 , D2O exchangeable), 4.63 (bs, H, HC=N), 5.33 (s,2H, 2×OH), 6.37 (s, H, Ar-H), 6.57(d, H, Ar-H J = 7.5 Hz), 7.48 (d, H, Ar-H J = 7.1 Hz),7.93 (d, 2H, Ar-H J = 7.6 Hz), 8.11 (d, 2H, Ar-H J = 7.8 Hz). Anal. calcd for C19H15ClN6O4S2: C, 46.48; H, 3.08; N, 17.12; Found C, 46.48; H, 3.08; N, 17.12; %
(E)-4-(4-(((5-(2-amino-6-hydroxyphenyl)-1,3,4-thiadiazol-2-yl)imino)methyl)-5-chloro-3-methyl-1H-pyrazol-1-yl)benzenesulfonamide (6n):
Yield: 62 %; m. p.: 178-180°C; IR (KBr) cm−1: 3372 (SO2NH2), 3172 (N-H), 3433(O-H), 1629 (C=N), 1534 (C=C), 1359 (SO2NH2), 1019 (C-N). 1H-NMR (300 MHz, DMSO-d6); δ 2.43 (s, 3H, Pyrazole-CH3), 3.54 (bs, 2H, SO2NH2 , D2O exchangeable), 4.72 (bs, H, HC=N), 5.39 (s,1H, OH), 6.28 (s,2H, Ar-N-H), 6.33 (s, 1H, Ar-H), 6.46(d, 2H, Ar-H J = 7.5 Hz), 7.01 (d, 1H, Ar-H J = 7.1 Hz),7.93 (d, 2H, Ar-H J = 7.6 Hz), 8.11 (d, 2H, Ar-H J = 7.8 Hz).13C-NMR (75 MHz, DMSO-d6); δ 13.70, 107.12, 109.3, 110.03, 114.34, 123.02, 127.54, 130.23, 133.03, 140.42, 146.08, 149.20, 156.03 (C-Pyrazolo), 160.25 (HC=N), 174.34 (C-thiadiazole). Anal. calcd for C19H16ClN7O3S2: C, 48.58; H, 3.29; N, 20.01; Found C, 48.38; H, 3.26; N, 20.29 %.
E)-4-(4-(((5-(4-aminophenyl)-1,3,4-thiadiazol-2-yl)imino)methyl)-5-chloro-3-methyl-1H-pyrazol-1-yl)benzenesulfonamide (6o):
Yield: 75%; m. p.: 163-165°C; IR (KBr) cm−1: 3371 (SO2NH2), 3173 (N-H), 1648 (C=N), 1535 (C=C), 1354 (SO2NH2), 1013 (C-N). 1H-NMR (300 MHz, DMSO-d6); δ 2.43 (s, 3H, CH3), 3.52 (bs, 2H, SO2NH2 , D2O exchangeable), 4.46 (bs, H, HC=N), 6.23 (s,2H, NH), 6.67 (s, 2H, Ar-H), 7.57(d, 2H, Ar-H J = 7.5 Hz), 7.93 (d, 2H, Ar-H J = 7.6 Hz), 8.11 (d, 2H, Ar-H J = 7.8 Hz). Anal. calcd for C19H16ClN7O2S2: C, 48.15; H, 3.40; N, 20.69; Found C, 48.12; H, 3.44; N, 20.78; %
Pharmacology
Anti-inflammatory activity
Carrageenan-induced rat paw oedema22method was used for the evaluation of in-vivo anti-inflammatory activity of synthesised compounds.Wistar rats were procured from Central Animal House of Jamia Hamdard, New Delhi (Registration no. 1141/CPCSEA) and were adapted to the room temperature in our laboratory. The animals were fasted overnight (12 h) of either sex weighing 150-200 g and divided into groups of six animals each. The Group- I served as control received; 0.5% w/v carboxymethyl cellulose (CMC), Group-II received standard drug celecoxib orally as a positive control at a dose level of 20mg/kg/body wt. and the test groups were administered orally with equamolar dosage of the synthesized compounds as the standard drug, After 1 hr, all animals were injected with 0.1 ml of 1% carrageenan solution (prepared in 0.9% of 0.1 ml of saline solution) in the sub plantar aponeurosis of left hind paw and the volume of paw was measured by using plethysmometer at interval of 3 h and 4 h post-carrageenan treatment.
Analgesic activity
The writhing test in mice was carried out using the method of Adeyemi23et al. The writhing effect was induced by intraperitoneal injection of 0.6% acetic acid (v/v) (80 mg/kg). Standard and tests compounds were administered orally at a dose of 20 mg/kg of body weight at an equimolar dosage to groups of six animals each, 30 min before chemical stimulus,Celecoxib was used as standard. The frequencies of muscle contractions were counted over a period of 20 min after acetic acid injection. The data represents the total number of writhes observed during 20 min and is expressed as writhing numbers.
Ulcerogenic activity
The test compounds having anti-inflammatory and analgesic activities comparable with the Celecoxib were further tested for their acute ulcerogenic risk evaluation according to the Cioli et almethod24.The dose of the tested and standard were used as three times of the dose used for the estimation of the anti-inflammatory activity, i.e. 60 mg/kg body weight.The control group received only 0.5% CMC. After the drug treatment, the rats were fed a normal diet for 17 h and then sacrificed. The stomach was removed and opened along the greater curvature. The tested and standard are compared with after opening of the gastric mucosa and the compounds did not cause any gastric ulceration and disruption of gastric epithelial cells at the above mentioned oral dose. Using microscope with a magnifying lens the effect of ulceration was examined. The mucosal damage in each stomach was assessed according to the following scoring system. The damage of the gastric mucosal damage was assessed according to the following scoring system: 0.5 redness, 1.0 spot ulcers, 1.5 hemorrhagic streaks, 2.0 ulcers < 3, but -5, 3.0 ulcers >5.
In-VitroCyclooxygenase(COX)InhibitionAssay
The selected synthesised compounds were accomplished there In-VitroCOX-II InhibitionAssay by previously reported method using enzymeimmunoassay (EIA)kit25. By measuring the formation of PGH2 during the biosynthetic process of arachidonic acid catalysed by COX-II enzymes, by the reduction of stannous chloride. The duplicate assay was performed as per the guidelines by the manufacturer. The absorption of diverseyellowcolour was measured by UV-visible spectrophotometer (EI 2371) at λ 412 nm. The intensity of the yellow colour is depends on the enzymatic reaction which is proportional to the prostaglandin tracer bound to the well and inversely to the amount in which present in well during incubation. In comparisons of tested compounds to various controlled incubation the percentage inhibition was measured. The concentration response curve was plotted for calculation of the concentration of test compounds that gives IC50 µM (COX-II).
Molecular Docking:
To predict binding modes of ligand to receptor and good biological activity on the basis of structures, a molecular docking studies were carried out using Glide extra precision (XP) Maestro 10.1Schrodinger, running on Linux 64 operating system based on X-ray crystal structure ofkey enzymes that are important for inflammatory process including COX-I (PDB: 1PGG) and COX-II (PDB: 3PGH).All the structure retrieved from protein data bank (www.rcsb.org).Molecular docking studies mainly involve selection and preparation of appropriate protein, grid generation, ligand preparation followed by docking and its analysis. The docking scoreand hydrogen bonds ad pi-pi interaction formed with the surrounding amino acids were used to predict their binding affinities and proper alignment of these compounds at the active site of the enzyme.
Results and Discussion
Chemistry
As per the scheme outlined in Figure (3),4-(5-chloro-3-methyl-4-(((5-phenyl-1,3,4-thiadiazol-2-yl) imino)methyl) -1H-pyrazol-1-yl) benzenesulfonamide derivatives were synthesized. Initially on refluxing equimolar mixture of phenyl hydrazine (1) and ethyl acetoacetate (2) at 110-120°C for four hour, 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one (3) was synthesized and further it was formylated using dry DMF in presence of POCl3, 5-chloro-3-methyl-1-phenyl-1H-pyrazole-4-carboxaldehyde (4) was attained20 (Kaushik et al., 2010).In another step using equimolar amount of substituted benzoic acid and semicarbazide werestirred in presence of POCl3 at 750C to get a substituted 5-phenyl-1, 3, 4-thiadiazol-2-amine21 (Tu et al., 2008).Finally formation of Schiff base as final compounds were obtained by refluxing the equimolar amount of 5-chloro-3-methyl-1-phenyl-1H-pyrazole-4-carboxaldehyde (4) and substituted 5-phenyl-1, 3, 4-thiadiazol-2-amine (5) In presence of catalytic amount of glacial acetic acid, achieved the title compound, 4-(5-chloro-3-methyl-4-(5-Substituted-1,3,4-thiadiazol-2-yl)imino)methyl)-1H-pyrazol-1-yl) benzene sulphonamide derivatives(6a-o). The structures of varied 4-(5-chloro-3-methyl-4-(5-Substituted-1,3,4-thiadiazol-2-yl)imino)methyl)-1H-pyrazol-1yl)benzenesulfonamide derivatives(6a-o) were elucidated by combined use of infrared (IR), 1H-NMR,13C-NMR and mass spectral (MS) data. In 1H-NMR spectra the D2O exchangeable broad singlet peak was observed around δ 7.12 ppm integrating two protons ascertained free SO2NH2 group in 4-formylpyrazoles.In IR spectra of (4) displayed two absorption bands in the region 3167cm-1 characteristic peak of N-H stretching signifying the presence of a free SO2NH2 group. In addition of IR spectra a band at 3079 cm-1 and 1667 cm-1 indicates C-H stretching of aldehydic and ketone group. The typical peak of SO2 was observed at 1327 and 1159 cm-1. A free aldehydic singlet peak was observed in 1H NMR spectra at δ 10.02-10.06 ppm along another singlet at δ 8.99-9.50 ppm for C5-H of pyrazole ring. Existence of CHO peak was further confirmed by a signal at δ 183.81ppm observed in the 13C NMR spectra. Formation of compounds (5) was confirmed by characteristic peak of NH2 group around sharp bands around 3283 and 3117 cm-1, stretching vibration of aromatic C-H and C-S peak were also observed at 1642 (C=N), 696 (C=S) cm-1. In a titled compounds (6a), disappearance of characteristics peak of NH2 and appearance of characteristic peak of stretching vibration at 1634 cm-1 due to formation of Schiff bases azomethine group (-CH=N-) was observed. It confirmed the formation of (E)-4-(5-chloro-3-methyl- 4-(((5-phenyl-1, 3, 4-thiadiazol -2-yl) methylene) amino)-1H-pyrazol-1-yl) benzene sulphonamide (6a).1H NMR spectra showed broad singlet peak of Schiff base of azomethine group (–CH=N-) at δ 4.61ppm.13C NMR spectra showed disappearance of aldehyde peak and appearance of (-CH=N-) at δ 161.91 ppm and at δ 173.34 ppm of thiadiazole confirmed the titled compounds. This was further supported by a mass spectrum of compound 6a (m/z 460.13, (M+2)).
In-vivo anti-inflammatory activity
With the help of docking analysis, synthesized eleven compounds were selected for anti-inflammatory, analgesic activity and ulcerogenic activity. Anti-inflammatory activity was done on Wistar rats (body weight 150-200 g) by Winter et al., method assuming rat paw edema inhibition test22.The paw oedema in rats was measured by plethysmometer. The dose of the compounds were selected at equimolar dose of 20 mg/kg of celecoxib. All the tests and reference compounds exposed anti-inflammatory activity in percentage inhibition in the ranging from 44.51 ± 6.19% to 74.59 ± 6.89% after 4 hr (Table-1, Figure-4). The structure activity relationship of the synthesized compounds was examined on the heart of the nature of substituted thiadiazole linked pyrazole benzene sulphonamide derivatives. The nature of the substituent varied on the aryl ring which is attached with 1, 3, 4-thiadiazole ring. The presence of hydroxyl substituent on aryl ring possesses very good in-vivo anti-inflammatory activity along with the halogen ring. The compounds (6c and 6e) with halogen substitution of chloro and bromo at para position bearing promising docking score and in-vivo anti-inflammatory activity (64.48±4.19 and60.87±7.28) and inhibition of intermediaries such as COX-I and COX-II. Substitution (6l) of hydroxy on aryl group at para position holds better dock score and in-vivo anti-inflammatory activity (58.73±3.17) and inhibition of intermediaries such as COX-I and COX-II. The substitution (6m) of hydroxy at both ortho and para, surprisingly increases the dock score and in-vivo anti-inflammatory (71.17±5.23 inhibition), analgesic and also acted to inhibit potentially COX-II as well as COX-I. The chloro substitution (6b) on aryl group at ortho position resulted in higher in-vivo anti-inflammatory activity (72.33± 3.83) than para position. The compounds containing CH3, OCH3 and NO2 group on aryl ring having low dock score as well as in-vivo and in-vitro biological activity.
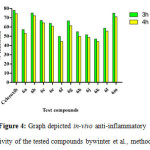 |
Figure 4: Graph depicted in-vivo anti-inflammatory activity of the tested compounds bywinter et al., method. Click here to View figure |
Table 1: Biological activities of (E)-4-(5-chloro-3-methyl-4-(((5-substituted-phenyl-1, 3,4-thiadiazol-2-yl)methylene)amino)-1H-pyrazol-1-yl)benzenesulfonamide derivatives 6 (a-o).
|
Compound |
Docking Scores
|
Anti-inflammatory activity≠(% inhibition ± SEM) | Analgesic activity#(% inhibition ± SEM After 4 h) | Ulcerogenic effect≠(Severity index ± SEM) | ||
| COX-I | COX-II |
3h |
4h |
|||
|
6a |
-5.99 |
— |
57.21 ± 6.87 | 53.18 ± 8.76 | 49.05 ± 3.75d | 0.58 ± 0.36b |
|
6b |
-8.26 |
-8.76 |
75.20± 4.38b | 72.33± 3.83* | 67.89 ± 2.33c | 0.77 ± 0.27b |
|
6c |
-8.72 |
-7.21 |
67.20± 4.90b | 64.48±4.19* | 45.25 ± 3.87d | 0.27 ± 0.71b |
|
6e |
-8.35 |
-7.40 |
63.98 ±6.07 | 60.87±7.28b | 53.45 ± 2.26d | 0.41 ± 0.10b |
|
6f |
-5.33 |
-3.65 |
49.98 ±5.77 | 44.51 ± 6.19 | 50.73 ± 1.87c | 0.47 ± 0.36b |
|
6g |
-8.88 |
-6.23 |
66.63± 4.94b | 61.49± 4.97a | 57.33 ± 1.87c | 0.54 ± 0.43b |
|
6h |
-6.25 |
— |
54.98 ± 6.47 | 49.87 ± 2.81 | 49.21 ± 1.87c | 0.59 ± 0.36b |
|
6j |
-8.30 |
— |
51.63 ± 6.89 | 48.72 ± 8.96 | 65.12 ± 1.20 c | 0.58 ± 0.36b |
|
6k |
-4.36 |
-6.01 |
47.05 ±6.60b | 44.31 ± 4.53 | 37.34 ± 4.15d | 0.50 ± 0.27b |
|
6l |
-8.76 |
-5.42 |
58.73±3.17 b | 55.57 ± 7.31 | 64.71 ± 2.90d | 0.39 ± 0.12a |
|
6m |
-8.57 |
-9.38 |
74.91 ± 7.69 | 71.17 ± 5.23 | 71.37 ± 1.67d | 0.79 ± 0.40b |
|
Celecoxib |
— |
— |
78.01 ± 3.75 | 74.59 ± 6.89 | 73.56 ± 1.25 | 0.93 ± 0.47 |
|
Control |
— |
— |
– | — | — | 0.00 ± 0.00 |
Relative to their respective standard and data were analyzed by ANOVA followed by Dunnett’s multiple comparison test for n = 6; aP < 0.05; bP < 0.01.
#Relative to normal and data were analyzed by paired Student’s t-test for n = 6; cP < 0.0001; dP < 0.005.
Table 2: In vitro COX-I and COX-II Enzyme Inhibition Data for compound
| Compounds | IC50 (µM) | Selectivity indexCOX-I/COX-II | |
| COX-I | COX-II | ||
| 6b | 217.10 | 3.20 | 67.81 |
| 6c | 109.65 | 2.4 | 45.68 |
| 6g | 129.71 | 5.2 | 24.94 |
| 6l | 183.68 | 5.03 | 36.51 |
| 6m | 239.73 | 3.6 | 66.38 |
| Celecoxib | 19.98 | 0.26 | 76.84 |
a Values are acquired using an ovine experiments were carried in duplicate and have less than 10% error.
Analgesic activity
The tested compounds displaying significant anti-inflammatory activity in comparison with standard were tested for their analgesic activity by the writhing test method 23. All the tested compounds exhibited analgesic activity in a range of 37.34 ± 4.15% to 69.37±1.67% inhibition, whereas standard drug celecoxib showed 73.56 ± 1.25% (Table-1, Figure-5).The compound with 2-chloro substitution (6b) showed very good analgesic activity (67.89 ± 2.33% inhibition) and the compound (6m) exhibited significant analgesic activity (71.37 ± 1.67% inhibition) as compared with reference drug celecoxib (73.56 ± 1.25% inhibition). The compound possesses potential anti-inflammatory and analgesic activities were further tested for their gastric ulceration activity according to Cioli et al. method at a dose of 60 mg/kg.
Ulcerogenic studies
The compounds having potential anti-inflammatory and analgesic activity was further evaluated their gastric ulceration activity Cioli et al24.Once accompanying with celecoxib, compounds 6b, 6c, 6e, 6l and 6m did not influence any gastric ulceration and rupture of the gastric mucosal layer (Table-1).
In-vitro COX Inhibition Studies
The selected compounds with promising in-vivo anti-inflammatory and analgesic activity were examined their in-vitroCOX Inhibition studies using enzymeimmunoassay (EIA)kitaccordingtoa previously described method25.The result of tested and reference drug were depicted in Table-2 figure-6. The results indicated that the compounds 6b and 6m exhibited significant inhibitory effect against COX-II which is compared with COX-I and it also possesses good selective index as compared to the reference drug. Selective profile of the selected compounds was calculated as ratios of (COX-I/COX-II) and it was compared with standard COX-II selective profile of celecoxib. The percentage inhibition of in-vitroCOX inhibition was depicted in figure-6.
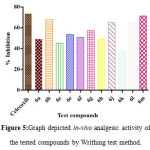 |
Figure 5: Graph depicted in-vivo analgesic activity of the tested compounds by Writhing test method. Click here to View figure |
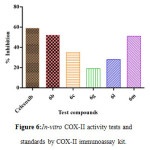 |
Figure 6: In-vitro COX-II activity tests and standards by COX-II immunoassay kit. |
ADME plays a crucial role in the design, screening and testing of the molecules for therapeutic intervention. To check the criteria of compounds for desirable pharmacokinetic properties, a QikProp study for prediction of ADME properties of the derivatives was performed using Schrodinger Maestro 10.1, running on Linux 64 operating system (QikProp. Version 3.6).26 Lipinski’s rule of five has been used to design and filter the compound that would likely to develop new clinical therapeutic agents and it is based on the observation that orally administered compounds have a MW < 500, logPo/w < 5, donorHB ≤ 5 and accptHB ≤ 10. Compounds violating more than one of these rules may have problems with oral bioavailability of compounds. Only two compounds 6e and 6f were violated Lipinski’s rule of five. From all these ADME parameters, it was concluded that most of the compounds followed Lipinski’s rule, making them potentially promising drug candidates for the treatment of inflammation as anti-inflammatory agents summarized in Table 3.
Table 3: ADME of compounds
|
Comp. |
aMW |
bPSA |
cDipole |
dSASA |
eDonor HB |
fAccpt HB |
glogPo/w |
hlogS |
iCNS |
% Human Oral Absorption |
jRule Of Five |
|
Range |
< 500 |
7– 200
|
1 –12.5 |
300 –1000 |
≤ 5 |
≤ 10 |
< 5 |
–6.5 – 0.5 |
+2 -2 |
> 80 good < 25 poor |
≤ 1 |
|
6a |
458.93 |
119.53 |
6.54 |
746.93 |
2 |
8.5 |
3.01 |
-6.27 |
-2 |
80.12 |
0 |
|
6b |
493.38 |
119.71 |
9.56 |
768.55 |
2 |
8.5 |
3.47 |
-6.90 |
-2 |
83.13 |
0 |
|
6c |
493.38 |
119.53 |
5.61 |
771.04 |
2 |
8.5 |
3.49 |
-6.99 |
-2 |
82.95 |
0 |
|
6d |
527.83 |
119.71 |
8.32 |
792.58 |
2 |
8.5 |
3.95 |
-7.62 |
-2 |
73.00 |
1 |
|
6e |
548.93 |
208.01 |
10.42 |
820.22 |
2 |
10.5 |
1.71 |
-6.48 |
-2 |
16.38 |
2 |
|
6f |
503.93 |
164.45 |
7.54 |
785.28 |
2 |
9.5 |
2.30 |
-6.41 |
-2 |
33.54 |
2 |
|
6g |
537.83 |
119.54 |
5.70 |
776.08 |
2 |
8.5 |
3.57 |
-7.10 |
-2 |
70.43 |
1 |
|
6h |
472.96 |
119.06 |
7.47 |
771.56 |
2 |
8.5 |
3.32 |
-6.69 |
-2 |
82.81 |
0 |
|
6i |
472.96 |
119.54 |
6.77 |
779.31 |
2 |
8.5 |
3.30 |
-6.82 |
-2 |
81.86 |
0 |
|
6j |
488.96 |
127.83 |
7.27 |
784.01 |
2 |
9.25 |
3.09 |
-6.47 |
-2 |
80.62 |
0 |
|
6k |
518.99 |
132.71 |
6.00 |
828.79 |
2 |
10 |
3.25 |
-6.81 |
-2 |
68.58 |
1 |
|
6l |
474.93 |
142.08 |
5.90 |
759.42 |
3 |
9.25 |
2.28 |
-6.05 |
-2 |
66.61 |
0 |
|
6m |
490.93 |
162.19 |
6.82 |
769.81 |
4 |
10 |
1.66 |
-5.78 |
-2 |
71.47 |
0 |
|
6n |
489.95 |
166.10 |
4.27 |
770.96 |
4.5 |
10.25 |
1.55 |
-5.69 |
-2 |
55.99 |
0 |
|
6o |
473.95 |
145.89 |
8.89 |
762.88 |
3.5 |
9.5 |
2.10 |
-6.00 |
-2 |
64.34 |
0 |
aMolecular weight of the molecule; bVan der Waals surface area of polar nitrogen and oxygen atoms and carbonyl carbon atoms; cComputed dipole moment of the molecule; dTotal solvent accessible surface area in square angstroms using a probe with a 1.4 Å radius; eEstimated number of hydrogen bonds that would be donated by the compound to water molecules in an aqueous solution; fEstimated number of hydrogen bonds that would be accepted by the compound from water molecules in an aqueous solution; gPredicted octanol/water partition coefficient; hPredicted aqueous solubility, log S. S in mol dm–3 is the concentration of the solute in a saturated solution that is in equilibrium with the crystalline solid; iCNS toxicity (+2 CNS active and -2 CNS inactive); jLipinski’s violations;
Molecular Docking
Docking study of all the synthesised compounds (6a-o) were performed using Glide extra precision (XP) Maestro 10.1 Schrodinger software 27 on COX-I (PDB: 1PGG) and COX-II (PDB: 3PGH) enzyme. The docking scores of the titled compounds with the active site of COX-I and COX-II is summarized in Table 1. Both the crystal structure of COX-I and COX-II was prepared for docking with the Protein Preparation Wizard workflow of Maestro that allows addition of hydrogen atoms which were subsequently minimized with OPLS-2005 force field and optimize the protonation state. The receptor grid was generated by applying a van der Waals radii of non-polar atoms, which decreases penalties for close contacts (scaling factor =1.00 and partial charge cut off = 0.25). Before docking calculation, all the compounds were subjected to ligand preparation with the LigPrep tool. Finally the ligand docking were run using receptor grid file and LigPrep out file in the Glide tool of Application view. It was observed that the compound 6m and 6b has shown less selectivity for COX-I and more for COX-II. The compounds also assume favourable orientation within the COX binding site. The 6m form a pi-pi interaction with TYR 355, while 6b form only hydrophobic interaction at active site of COX-I as shown in docked pose Figure-7.The compound 6m and 6b formed strong hydrogen bonds with ARG 120 and TYR 355 respectively at active site of COX-II as shown in docked pose Figure-7.The docking studies of compound 6m and 6galso revealed that the presence of linker moiety between two heterocyclic rings are important for hydrogen bonding with ARG 120 and TYR 355 respectively at COX-II site. The binding orientation of titled compounds was found to be same as co-crystal ligand. In COX-II, the smaller size of the valine (VAL 523) side chain coupled with the conformational changes at TYR 355 opens up the hydrophobic segment of the new pocket. Because of substitution of bulkier isoleucine (ILE) of COX-I to smaller valine (VAL) at position 523 lead to inhibition of COX-II by compounds.
 |
Figure 7: Docked Pose of (a)Compound 6m,green colour(b)Compound 6b,turquoise colour, represented as tube in the binding site of COX-1 showing pi-pi interaction (turquoise dash lines) with TYR 355 and Docked Pose of (c)Compound 6m,lime green colour(d)Compound 6b,turquoise colour, represented as tube in the binding site of COX-2 showing hydrogen bond interaction (red dash lines) with ARG 120 and TYR 355 and pi-pi interaction (turquoise dash lines) with TYR 115 and TYR 355. Click here to View figure |
Conclusion
It can be concluded that we have synthesized thiadiazole linked pyrazole benzene sulphonamide derivatives (6a-o) and characterized by IR NMR and Mass spectral data and estimated their anti-inflammatory, analgesic activity and ulcerogenic activity along with in-vitro COX-II inhibitory activity. The compounds 6b, 6c, 6i, 6l and 6m exhibited significant anti-inflammatory and analgesic activity without showing any gastric ulceration. The COX-II inhibitory potential represented that the compound 6b and 6m exhibited very good anti-inflammatory and analgesic activity with selective index of (SI-67.81 and 66.38 respectively), which was compared with reference drug of Celecoxib (SI- 76.84). The molecular docking and in-silico computational study revealed that the compound 6b and 6m has very good binding affinity with amino acid ARG 120 and TYR 355for COX-II and also embraces a prominent pharmacokinetic profile. Hence the compounds 6b and 6m were selected as promising lead candidates for further development of selective inhibition for COX-II and anti-inflammatory activity.
Acknowledgement
The authors are thankful to Jamia Hamdard New Delhi, India for providing facility for research work. The author are thankful to IIT Delhi and Jamia Hamdard for spectral and elemental studies. One of the authors (Md Jahangir Alam) expresses thanks to University Grant Commission (UGC) New Delhi, India for the award of Moulana Azad National Fellowship as financial assistance.
Conflict of interest:– The authors declares no Conflict of Interest.
Reference
- Nathan C.,Nat.2002.420: 846-852.
- Vergelli C., Giovannoni M.P., Pieretti S. A., GiancarloD., Dal Piaz V.,Bioorg. Med. Chem.2007. 15, 563-5575.
- Williams M., Kogaluc E.A., Arneric S.P.,J. Med. Chem. 1999.42, 1481.
- Wada M., DeLong C.J., Hong Y.H., Rieke C.J., Song I., Sidhu R.S., Yuan C., Warnock M., Schmaier A.H., Yokoyama C., Smyth E.M., Wilson S.J., FitzGerald G.A., Garavito R.M., Sui de X., Regan J.W., Smith W.L.,J. Biol. Chem.2007. 28, 22254-22266.
- Abbas S.E., Awadallah F.M., Ibrahin N.A., Said E.G., Kamel G.K.,Eur. J. Med. Chem. 2012. 53, 141.
- Salgin-Goksen U., Gokhan-Kelekci N., Goktas O., Koysal Y., Kilic E., Isik S., Aktay G., Ozalp M., Bioorg. Med. Chem. 2007. 15, 5738-5751.
- Tewari A.K., Mishra A.,Bioorg. Med. Chem.2001. 3, 715.
- Bornet R.F., Williams D.A., Lemke T.L., Williams D.A., 5th ed, Lippincott Williams and Wilkins, 2002.751.
- Rubin E., Lazar D., Associaton of Academic Health Centers. 2009.
- Saleem S., Ahmad P., Al-Harb N.O., Shaharyar M.A., Rahman R.U., Iqbal M., Khusroo M.J., Ahmad K., Imam F., Alam M.J.,Mol. Cell. Biochem.2013. 384, 147-153.
- Dawane B.S., Konda S.G., Mandawad G.G., Shaikh B.M.,Eur. J. Med. Chem. 2010.45,387-392.
- Mui M.S., Siew B.N., Buss A.D., Crasta S.C., Kah L.G., Sue K.L.,Bioorg. Med. Chem. Lett.2002.12, 679-699.
- Ahsan M.J., Samy G.J., Dutt K.R., Agrawal U.K., Shankar B., Vyas S., Kaur R., Yadav G., Bioorg. Med. Chem. Lett.2011,21, 4451-53.
- Sridhar R., Perumal P.T., Etti S., Shanmugam G., Ponnuswamy M.N., Prabavathy V.R., Mathivanan N.,Biorg. Med. Chem. Lett. 2004.14, 6035-6040.
- Alam M.J., Ahsan M.J., Alam O., Khan S.A.,Lett. Drug. Des. Discov. 2013. 10, 776-782.
- Patrick D.A., Bakunov S.A., Bakunova S.M., Wenzler T., Tidwell R.R.,Bioorg. Med. Chem.1422.20, 559-576.
- Turan-Zitouni G., Chevallet P., Kiliç F.S., Erol K.,Eur J Med Chem. 2000.35, 635-641.
- Bilgin A.A., Palaska E., Sunal R.,Drug. Res.1993.43, 1041-1044.
- Afzal M., Gupta G., Kazmi I., Rahman M., Alam M.J., Hakeem K.R., Pravez M., Gupta R., Afzal O., Anwar F.,Fitoterapia. 2012.83, 853-858.
- Kaushik D., Khan S.A., Chawla G., Kumar S.,Eur. J. Med. Chem.2010.45, 3943-3949.
- Tu G.G., Li S.H., Huang H.M., Li G., Xiong F., Mai X., Kuang B.H., Xu W.F., Zhu H.W.,Bioorg Med Chem.2008.16, 6663-6668.
- Winter A.C., Risley T.E., Nuss W.G.,Proc. Soc. Exp. Biol. Med.1962.3, 544-547.
- Adeyemi O., Okpo O.S., Okpaka O.,J. Ethnopharmacol. 2004.90, 45-48.
- Cioli V., Putzolu S., Rossi V., Barcellona P.S., Corradino C.,Toxicol Appl Pharmacol.1979. 50, 283-289.
- UddinM.J., Rao P.N.P., McDonald R., Knaus E.E., J.Med.Chem.2004.47, 6108-6111.
- Schrodinger, Maestro, QikProp study for prediction of ADME properties. Version 10.1, LLC, New York, NY, 2015.
- Schrodinger, Maestro, Version 10.1, LLC, New York, NY, 2015.

This work is licensed under a Creative Commons Attribution 4.0 International License.









