Surprises in the Study of Ruthenium-catalyzed Stereo- and Chemoselective Aldolizations
Elahe Keshavarz,1,* Khalil Tabatabaeian2, Manouchehr Mamaghani2 and Nosrat O. Mahmoodi2
1Department of Sciences, Farhangian University, P.O. Box 1998963341, Rasht, Iran.
2Department of Chemistry, Faculty of Sciences, Guilan University, P.O. Box 41335-1914, Rasht, Iran.
Corresponding Author E-Mail: keshavarz@guilan.ac.ir
DOI : http://dx.doi.org/10.13005/ojc/310441
Article Received on :
Article Accepted on :
Article Published : 20 Oct 2015
A convenient and diastereoselective method was developed for the synthesis of aldol derivatives in the presence of a catalytic amount of RuCl3.nH2O under solvent-free conditions. Aldol adducts were obtained in good yields and with high chemoselectivity in short reaction times. In this protocol, aromatic and heteroaromatic aldehydes readily participate as electrophilic cross-aldol partners with a range of cycloalkanones as ketone donors.
KEYWORDS:catalytic aldol reaction; diastereoselective aldolization; green synthesis
Download this article as:| Copy the following to cite this article: Keshavarz E, Tabatabaeian K, Mamaghani M, Mahmoodi N. O. Surprises in the Study of Ruthenium-catalyzed Stereo- and Chemoselective Aldolizations. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Keshavarz E, Tabatabaeian K, Mamaghani M, Mahmoodi N. O. Surprises in the Study of Ruthenium-catalyzed Stereo- and Chemoselective Aldolizations. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12008 |
Introduction
Apart from the economic and environmental aspects, the use of catalysts has shown their attractiveness due to their activity and selectivity.1-5 Metal-catalyzed reactions have made a great contribution to the recent growth of organic synthesis and a variety of synthetic methods have been reported using transition metal complexes in stoichiometric or catalytic amounts.6,7 Among the transition metal Lewis acids, ruthenium salts and complexes display remarkable properties.8,9
During the last decade, molecular ruthenium catalysts have promoted tremendous developments in organic synthesis methodology and polymer science, and revealed novel activation processes. Ruthenium catalysis constitutes an emerging field for the selective preparation of fine chemicals. This is due to the availability of a large number of well-defined and stable ruthenium precatalysts offering several possible oxidation states. They usually tolerate functional groups and have revealed catalytic activities for a wide range of chemical transformations with atom economy.10-15
On the other hand, carbon-carbon bond formation is the essence of organic synthesis and its variants have been used extensively in many important syntheses.16-19 The aldol reaction is a powerful method for forming C-C bonds.20-24 The reaction has proven to be a powerful and general method for the stereocontrolled construction of β-hydroxy ketone derivatives and has relevant application in the synthesis of carbohydrates, amino sugars, steroids, natural heteroatomic molecules, fine chemicals and pharmaceuticals. There are several methods to synthesized aldol products. However, only few reports described the use of catalyst to perform the aldol reactions in the simple conditions.
Over the last thirty years, seminal research from the laboratories of Evans,25 Heathcock,26,27 Masamune28 and Mukaiyama29 have established this venerable reaction as the principal chemical method for the stereoselective construction of complex polyol architecture. Also, many efforts have been addressed towards the synthesis of organocatalysts that have produced systems able to afford very good stereoselectivities. However, the achievement of a versatile organocatalyst, able to work with low catalyst loading remains a challenge.30-34
These observations prompted us to synthesize a series of aldol derivatives with complete diastereoselectivity using RuCl3.nH2O as an efficient catalyst via a convenient one-pot reaction under solvent-free conditions. It is worth noting that only a little amount of ruthenium catalyst was used, which represents a significant improvement over the conventional aldol reaction.
Material and Methods
General
All solvents, organic and inorganic compounds were purchased from Merck and Fluka and used without further purification. All reactions were followed by TLC with detection by UV light. IR spectra were recorded on Shimadzu FTIR-8400S spectrometer. 1H NMR spectra were obtained on a Bruker DRX-500 Avance spectrometer and 13C NMR were obtained on a Bruker DRX-125 Avance spectrometer. Samples were analyzed in CDCl3, and the chemical shift values are reported in ppm relative to TMS (tetramethylsilane) as the internal reference. Elemental analyses were made by a Carlo-Erba EA1110 CHNO-S analyzer and agreed with the calculated values. The isolation of pure products was carried out via preparative thin layer chromatography (silica gel 60 GF254, Merck).
Typical procedure for ruthenium-catalyzed aldol reaction
A mixture of aldehyde (1 mmol), ketone (3 mmol), RuCl3.nH2O(0.02 mmol) and KOH (28 mg, 0.5 mmol) was stirred at 15 °C and monitored by TLC. After the indicated reaction time, the reaction mixture was purified by thin layer chromatography (silica gel, EtOAc-petroleum ether, 4:12) providing the aldol adduct.
Selected Spectral Data of the Products
Product (3aa)
Yellow oil; FT-IR (neat) (υmax/cm-1): 1666, 3415. Only syn isomer was isolated. 1H NMR (500 MHz, CDCl3): δH(ppm) 1.69-2.65 (m, 7H), 2.52 (s, 3H), 3.03 (d, J = 3.2 Hz, OH), 5.39 (m, 1H), 7.28 (m, 4H). 13C NMR (125 MHz, CDCl3): δC(ppm) 12.2, 14.0, 14.9, 41.1, 58.1, 72.2, 126.5, 128.5, 133.5, 135.2, 218.4. Anal Calcd for C13H16O2S (236): C, 66.10; H, 6.77; S, 13.55. Found: C, 66.10; H, 6.79; S, 13.56.
Product (3ba)
Yellow oil; FT-IR (neat) (υmax/cm-1): 1656, 3412. Only syn isomer was isolated. 1H NMR (500 MHz, CDCl3): δH(ppm) 2.50-1.74 (m, 7H), 2.28 (s, 3H), 4.73 (s, OH), 5.61 (s, 1H), 6.81 (d, J = 5.0 Hz, 1H), 7.24 (d, J = 5.0 Hz, 1H). 13C NMR (125 MHz, CDCl3): δC(ppm) 16.2, 23.4, 28.8, 44.0, 57.1, 58.2, 123.8, 125.5, 124.6, 138.3, 143.3, 217.1. Anal Calcd for C11H14O2S (210): C, 62.85; H, 6.66; S, 15.23. Found: C, 62.86; H, 6.66; S, 15.24.
Product (3bb)
Yellow oil; FT-IR (neat) (υmax/cm-1): 1667, 3345. Only syn isomer was isolated. 1H NMR (500 MHz, CDCl3): δH(ppm) 2.71-1.59 (m, 9H), 2.27 (s, 3H), 4.08 (d, J = 1.6 Hz, OH), 5.52 (s, 1H), 7.24-7.19 (m, 2H). 13C NMR (125 MHz, CDCl3): δC(ppm) 16.4, 23.6, 28.8, 42.1, 44.5, 58.2, 124.8, 125.5, 125.8, 136.3, 143.1, 217.2. Anal Calcd for C12H16O2S (224): C, 64.28; H, 7.14; S, 14.28. Found: C, 64.31; H, 7.16; S, 14.28.
Product (3cc)
Yellow oil; FT-IR (neat) (υmax/cm-1): 1674, 3465. Only syn isomer was isolated. 1H NMR (500 MHz, CDCl3): δH(ppm) 2.92-1.54 (m, 11H), 2.44 (s, 3H), 3.37 (d, J = 3.9 Hz, OH), 5.25 (s, 1H), 6.60 (d, J = 2.4 Hz, 1H), 6.72 (d, J = 3.2 Hz, 1H). 13C NMR (125 MHz, CDCl3): δC(ppm) 15.2, 23.6, 25.3, 28.4, 28.6, 44.0, 57.8, 71.5, 124.5, 124.6, 138.6, 143.3, 217.2. Anal Calcd for C13H18O2S (238): C, 65.54; H, 7.56; S, 13.44. Found: C, 65.53; H, 7.56; S, 13.44.
Product (3dc)
Yellow oil; FT-IR (neat) (υmax/cm-1): 1678, 3366. Only syn isomer was isolated. 1H NMR (500 MHz, CDCl3): δH(ppm) 2.91-1.50 (m, 11H), 3.35 (d, J = 3.5 Hz, OH), 5.35 (s, 1H), 6.63-6.86 (m, 3H). 13C NMR (125 MHz, CDCl3): δC(ppm) 23.1, 25.6, 28.4, 29.1, 44.3, 57.8, 71.6, 124.5, 124.5, 138.5, 141.3, 217.2. Anal Calcd for C12H16O2S (224): C, 64.28; H, 7.14; S, 14.28. Found: C, 64.29; H, 7.14; S, 14.28.
Results and Discussion
Recently, we have examined an efficient protocol for the cross aldol reaction in the presence of RuCl3.nH2O/PPh3. In this protocol, the reaction of 1-(thiophen-2-yl)ethanone with aldehydes resulted aldol adducts in good yields without the formation of any side product.35 The study of the RuIII/PPh3-promoted aldol reaction urged us to investigate the aldolization of aryl alkyl ketones with aromatic and heteroaromatic aldehyds in the presence of RuCl2(PPh3)3. RuII-catalyzed cross aldol reaction proceeded at 80°C, furnishing a wide variety of β-hydroxy ketones with moderate diastereoselectivities.36 This led us to discover unexpected effects of the nature of chiral ligand on the RuIII-catalyzed aldolization of aromatic aldehyds with ketones. In this method, the aldol reaction proceeded smoothly, affording the corresponding product in good yields and complete diastereoselectivities.37
In the present protocol, due to the importance of development of new diastereoselective methodologies in the synthesis of organic compounds with high chemoselectivity, we performed RuIII-catalyzed aldol reactions using various aldehydes and cycloalkanones without the use of chiral system in good yields with complete diastereoselectivity. In this method, all reactions were carried out by simply adding the ruthenium catalyst (RuCl3.nH2O) to a neat solution containing aldehyde and cycloalkanone at 15°C when the molar ratio of cycloalkanones and aldehydes was 3 (Scheme 1). The successful results of RuIII-catalyzed aldol reactions are given in Table 1.
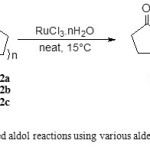 |
Scheme 1: RuIII-catalyzed aldol reactions using various aldehydes and cycloalkanones |
Table 1: Ruthenium-catalyzed cross aldol reactions of aldehydes with cycloalkanones 3aa-ec.
| Entrya | R | Aldol | Yield (%)b | Time (h) |
| 1 | 1a 4-MeSC6H4 | 3aa | 81 | 5 |
| 2 | 1b 3-Methylthiophen-2-yl | 3ba | 86 | 1.5 |
| 3 | 1c 5-Methylthiophen-2-yl | 3ca | 83 | 1 |
| 4 | 1d Thiophen-2-yl | 3da | 91 | 3 |
| 5 | 1a 4-MeSC6H4 | 3ab | 79 | 4.5 |
| 6 | 1b 3-Methylthiophen-2-yl | 3bb | 76 | 2.5 |
| 7 | 1c 5-Methylthiophen-2-yl | 3cb | 77 | 3 |
| 8 | 1a 4-MeSC6H4 | 3ac | 72 | 4.5 |
| 9 | 1b 3-Methylthiophen-2-yl | 3bc | 80 | 2.5 |
| 10 | 1c 5-Methylthiophen-2-yl | 3cc | 78 | 2.5 |
| 1112c | 1d Thiophen-2-yl1e 4-NO2C6H4 | 3dc3ec | 7355 | 25 |
aAll products were characterized by 1H NMR, 13C NMR and IR. b Yields after purification by chromatography. c Identified by comparison with authentic sample.38
By comparing the entries, for the heteroaromatic aldehydes 1 b-d, the reaction could complete within 1-3 h. For the aromatic aldehyde, much longer reaction time was required. Although the activity of catalyst decreased when using aromatic aldehyde, it can be used at 2 mol% loading and only a slight excess of the ketone with respect to the aldehyde is required (3eq). This result provided a remarkable contrast to similar reactions, where a large excess of the donor carbonyl compound is mandatory to perform the reaction.37, 39-46
As far as the diastereomeric ratios are concerned, complete selectivities in favor of the syn products were observed in all the cases 3aa-ec. Configurational assignment of diastereoisomers was attributed by NOESY, 1H NMR and IR.
It is a well understood phenomenon that the lower values of the 1H NMR coupling constants of the carbinol protons than 1 Hz, for example 3cc, along with very weak correlation between the 1-H and 2-H (Figure 1) in the NOESY spectrum clearly indicate the relative stereochemistry of the aldol adduct in favor of the syn geometry. Also, in the IR spectrum of 3cc, the broad band of OH was observed with a maximum at 3500 cm-1, which indicates the existence of hydrogen bonding of aldol adduct (Figure 2). In fact, the structure has been fixed in a special stereochemistry which dominated by hydrogen bonding observed in IR spectra and the tendency to minimize the steric crowding between the 5-Methylthiophen-2-yl and cycloheptanone rings. These results illuminate that 1-C, 2-C, 1-H and 2-H has the same chemical environment, that is to say 3cc is exclusively of syn stereochemistry.
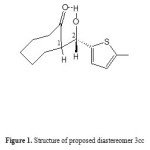 |
Figure 1: Structure of proposed diastereomer 3cc |
 |
Figure 2: IR spectrum of 3cc Wavenumber (cm-1) Click here to View figure |
The fact that all of the products shared almost the same NMR and IR patterns suggests the stereochemistry of these compounds to be identical.
Conclusion
In brief, the direct aldol reaction of various cycloalkanones with heteroaromatic and aromatic aldehydes using catalytic amount of RuCl3.nH2O without the use of chiral ligand proceeded smoothly under extremely mild conditions to give the corresponding aldol adducts in moderate to good yields with complete diastereoselectivity. The reaction can efficiently proceed at 15 °C with a small excess of ketone. From a practical and economic point of view this reaction condition is convenient with respect to most of other protocols where a large excess of ketone is used. Also, the chemoselective aldolization has shown good generality and enough activity without using any organic solvent. Further investigations focusing on the mechanism and full scope of related systems are currently underway and
Acknowledgements
The authors would like to acknowledge the partial support of this work by Guilan University Research Council. The author (E.K.) sincerely thanks Farhangian University officials for all the hard work and efforts on the development of University, their encouragement to advanced research and the promotion of science.
References
- Masters, C. Homogeneous Transition Metal Catalysis-A Gentle Art, Chapman and Hall Ltd. (1981).
- Meijere, A. D.; Diederich, F. Metal-Catalyzed Cross-Coupling Reactions, Vol. 2, 2nd edn. Wiely-VCH, New York, (2004).
- Dou, X.-Y.; Wang, J. Q.; Du, Y.; Wang, E.; He, L.-N. Synlett 2007, 3058-3062.
- Hossein Nia, R.; Mamaghani, M.; Tabatabaeian, K.; Shirini, F.; Rassa, M. Acta Chim. Slov. 2013, 60, 889-895..
- Qian, S.-S.; Cheng, X.-S.; You, Z.-L.; Zhu, H.-L. Acta Chim. Slov. 2013, 60, 870–874.
- Madern, N.; Talbi, B.; Salmain, M. Appl. Organomet. Chem. 2013, 27, 6-12.
- Kidwai, M.; Jain, A. Appl. Organomet. Chem. 2012, 26, 528-535.
- Murahashi, S.-I. Ruthenium in Organic Synthesis, Wiely-VCH, New York, (2004).
- Murai, S. Activation of Unreactive Bonds and Organic Synthesis, Springer, (1999).
- Tabatabaeian, K.; Heidari, H.; Mamaghani, M.; Mahmoodi, N. O. Appl. Organomet. Chem. 2012, 26, 56-61.
- Tabatabaeian, K.; Mamaghani, M.; Mahmoodi, N. O.; Khorshidi, A. Tetrahedron Lett. 2008, 49, 1450-1454.
- Tabatabaeian, K.; Mamaghani, M.; Mahmoodi, N. O.; Khorshidi, A. Catal. Commun. 2008, 9, 416-420.
- Tabatabaeian, K.; Mamaghani, M.; Mahmoodi, N. O.; Khorshidi, A. J. Mol. Catal. A. 2007, 270, 112-116.
- Tabatabaeian, K.; Mamaghani, M.; Mahmoodi, N. O.; Khorshidi, A. Can. J. Chem. 2006, 84, 1541-1545.
- Tabatabaeian, K.; Keshavarz, E.; Mamaghani, M.; Mahmoodi, N. O. Org. Chem. Int. 2011, ID 325291, 5 pages.
- Stodulski, M.; Mamińska, A.; Mlynarski,J. Tetrahedron: Asymmetry, 2011, 22, 464-467.
- Gotoh, R.; Yamanaka,M. Molecules 2012, 17, 9010-9022.
- El-Sepelgy, O.; Schwarzer, D.; Oskwarek, P.; Mlynarski, J. Eur. J. Org. Chem. 2012, 2724-2727.
- Woyciechowska, M.; Forcher, G.; Buda, S.; Mlynarski, J. Chem. Commun. 2012, 48, 11029- 11031.
- Nicolas, C.; Pluta, R.; Pasternak-Suder, M.; Martin, Q. R.; Mlynarski, J. Eur. J. Org. Chem. 2013, 1296-1305.
- Kawato, Y.; Iwata, M.; Yazaki, R.; Kumagai, N.; Shibasaki, M. Tetrahedron, 2011, 67, 6539-6546.
- El-Sepelgy, O.; Mlynarski, J. Adv. Synth. Catal. 2013, 355, 281-286.
- Geary, L. M.; Hultin, P. G. Tetrahedron: Asymmetry 2009, 20, 131-173.
- Yamanaka, M.; Hoshino, M.; Katoh, T.; Mori, K.; Akiyama, T. Eur. J. Org. Chem. 2012, 4508-4514.
- Evans, D. A.; Bartroli, J.; Shih, T. L. J. Am. Chem. Soc. 1981, 103, 2127-2129.
- Danda, H.; Hansen, M. M.; Heathcock, C. H. J. Org. Chem. 1990, 55, 173-181.
- Heathcock, C. H. Science 1981, 214, 395-400.
- Masamune, S.; Choy, W.; Kerdesky, F. A. J.; Imperiali, B. J. Am. Chem. Soc. 1981, 103, 1566- 1568.
- Mukaiyama, T.; Banno, K. Narasaka, K. J. Am. Chem. Soc. 1974, 96, 7503-7509.
- Chandrasekhar, S.; Johny, K.; Reddy, C. R. Tetrahedron: Asymmetry 2009, 20, 1742-1745.
- Rani, R.; Peddinti, R. K. Tetrahedron:Asymmetry 2010, 21, 775-779.
- Chen, F.; Huang, S.; Zhang, H.; Liu, F.; Peng, Y. Tetrahedron 2008, 64, 9585-9591.
- Siyutkin, D. E.; Kucherenko, A. S.; Zlotin, S. G. Tetrahedron 2009, 65, 1366-1372.
- Tzeng, Z.-H.; Chen, H.-Y.; Reddy, R. J.; Huang, C. T.; Chen, K. Tetrahedron 2009, 65, 2879- 2888.
- Tabatabaeian, K.; Mamaghani, M.; Mahmoodi, N. O.; Keshavarz, E. Int. J. Inorg. Chem. 2010, ID 621376, 4 pages.
- Tabatabaeian, K.; Mamaghani, M.; Mahmoodi, N. O.; Keshavarz, E. Arkivoc 2009, II, 68-75.
- Tabatabaeian, K.; Keshavarz, E.; Mamaghani, M.; Mahmoodi, N. O. Arkivoc 2010, IX,155-162.
- Luo, S.; Mi, X.; Zhang, L.; Liu, S.; Xu, H.; Cheng, J.-P. Tetrahedron 2007, 63, 1923-1930.
- Jia, Y.-N.; Wu, F.-C.; Ma, X.; Zhu, G.-J.; Da, C.-S. Tetrahedron Lett. 2009, 50, 3059-3062.
- Miura, T.; Yasaku, Y.; Koyata, N.; Murakami, Y.; Imai, N. Tetrahedron Lett. 2009, 50, 2632-2635.
- Kan, S.-S.; Li, J.-Z.; Ni, C.-Y.; Liu, Q.-Z.; Kang, T. R. Molecules 2011, 16, 3778-3786.
- Keshavarz, E.; Tabatabaeian, K.; Mamaghani, M.; Mahmoodi, N. O. Curr. Chem. Lett. 2012, 1, 91-94.
- Shanmuganathan, S.; Greiner, L.; Domínguez de María, P. Tetrahedron Lett. 2010, 51, 6670- 6672.
- Inoue, H.; Kikuchi, M.; Ito, J.-I.; Nishiyama, H. Tetrahedron 2008, 64, 493-499.
- Chen, Y.-L.; Li, W.; Liu, Y.; Guan, Z.; He, Y.-H. J. Mol. Catal. B. 2013, 87, 83-87.
- Shen, C.; Lia, H.; Shen, F.; Zhang, P. Catal. Commun. 2013, 41, 106-109.

This work is licensed under a Creative Commons Attribution 4.0 International License.









