Study of Physical and Colloid-Chemical Properties of Acrylic Polyelectrolytes of "M-PAA" Series And Their Modification
N.O Dzhakipbekova, A. B Isa, M. F Fatkullina, and E. O Dzhakipbekov
M. Auezov South-Kazakhstan State university, Shymkent city, Kazakhstan, 160019, Corresponding Author Email: dzhakipbekova@mail.ru
DOI : http://dx.doi.org/10.13005/ojc/310421
Article Received on :
Article Accepted on :
Article Published : 04 Nov 2015
The aim of this study is to search for the best basic technology to replace the base in the metallization of dielectrics. We studied conducting polymers – acrylic polyelectrolytes. Polyelectrolytes include high molecular weight compounds containing macromolecules and ionogenic groups. Experimental studies have shown that the rational use of HSP for the regulation of colloid-chemical processes in the production should take into account the functional structure of the polymer, its conformational state of macromolecules in solution, the degree of association, dissociation of functional groups, and other factors, which necessitates studying the physical and colloidal chemical characteristics of HSP solutions depending on the concentration.
KEYWORDS:polyelectrolytes; ethanolamine; monoethanolamine; viscosity; density
Download this article as:| Copy the following to cite this article: Dzhakipbekova N O, Isa A. B, Fatkullina M. F, Dzhakipbekov E. O. Study of Physical and Colloid-Chemical Properties of Acrylic Polyelectrolytes of "M-PAA" Series and Their Modification. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Dzhakipbekova N O, Isa A. B, Fatkullina M. F, Dzhakipbekov E. O. Study of Physical and Colloid-Chemical Properties of Acrylic Polyelectrolytes of "M-PAA" Series and Their Modification. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12179 |
Introduction
The aim of this study is to search for the best basic technology to replace the base in the metallization of dielectrics. We studied conducting polymers – acrylic polyelectrolytes. Polyelectrolytes include high molecular weight compounds containing macromolecules and ionogenic groups. Polyelectrolytes can be of natural or synthetic origin. Among natural compounds, the most known are: proteins, nucleic acids and polysaccharides such as alginic acid, heparin and the like [1].
Isolation of reagents from raw materials of plant and animal origin often costs more than the production of synthetic polymers. Therefore, along with the polymers of natural origin and their modification products synthetic reagents are currently becoming more common, the use of which allows the synthesis of high molecular weight soluble polyelectrolytes (HSP) with desired properties and composition.
Experimental studies have shown that the rational use of HSP for the regulation of colloid-chemical processes in the production should take into account the functional structure of the polymer, its conformational state of macromolecules in solution, the degree of association, dissociation of functional groups, and other factors, which necessitates studying the physical and colloidal chemical characteristics of HSP solutions depending on the concentration.
Polyelectrolytes are: the first sample is a hydrolyzed polyacrylamide modified by sulfanol (S-PAA), the second sample is a hydrolyzed polyacrylamide modified by monoethanolamine (MEA-PAA), the third sample is a hydrolyzed polyacrylamide modified by hydrogen peroxide (HP-PAA) . They were synthesized on the basis of polyacrylamide in the medium of sodium hydroxide in the presence of modifiers.
In order to determine their macromolecular, surfactant and polyelectrolyte character the dependence of the viscosity, pH, conductivity, surface tension on concentrations (0.01-5%) were investigated, the isoelectric point for the three polymers was determined, the properties of polymers at the interface were examined.
The results of measurement of viscosity (η), electric conductivity (χ), pH, optical density (D) versus concentration are shown in Table 1. They were compared with the solutions of the known K-4 polyelectrolyte.
Table 1 shows that at similar concentrations of PE (from 0.01 to 5%), the relative viscosity of “S-PAA” “MEA PAA” and “HP-PAA” polymer solutions is considerably higher than that of K-4 polyelectrolyte. In our opinion, this may be due to the increase in molecular weight as a result of the interaction of ethanolamines with K-4, which is confirmed by IR spectroscopic data. Furthermore, interaction of ethanolamines can occur according to the mechanism of bridged imide groups and hydrogen bonds on alcoholic OH groups between adjacent K-4 macromolecules formation, which leads to the solution viscosity increase.
Specific viscosity curves show the increase of the specific viscosity with the increasing concentrations of HSP in the solution that is also characteristic of the growth of indirect interaction of the macromolecules in all polyelectrolyte solutions. This indicates that the polymers have different molecular weights and molecular coil dimensions.
Table 1: Change of colloid-chemical properties of the HSP solutions depending on their concentration
| Polyelectrolyte concentration | relative viscosity ηrel | Specific viscosity ηsp | Reduced viscosity ηrel/s | electrical conductivity χ | рН | optical density D |
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
|
К-4 |
||||||
|
0,01 0,03 0,05 0,1 0,3 0,5 1,0 3,0 5,0 |
1,27 1,52 1,81 2,7 4,7 6,63 10,7 12,4 19,7 |
0,25 0,55 0,89 1,8 3,9 5,7 9,7 11,5 18,9 |
25,6 17,5 17,1 16,8 13,6 11,4 9,7 3,8 3,2 |
4,5 9,3 17,8 38,6 114,3 151,3 301,6 921,6 1565,9 |
10,54 10,78 10,93 11,39 11,43 11,75 12,56 12,89 13,66 |
0,003 0,005 0,007 0,04 0,08 0,2 0,4 0,5 0,5 |
|
S-PAA |
||||||
|
0,01 0,03 0,05 0,1 0,3 0,5 1,0 3,0 5,0 |
1,43 1,98 2,37 3,6 5,69 7,73 13,49 14,45 20,92 |
0,43 0,98 1,45 2,6 4,7 6,5 12,46 13,89 16,78 |
42,4 32,9 26,8 25,4 15,4 13,2 12,1 4,9 3,3 |
3,9 7,6 11,8 39,2 108,2 135,6 256,1 731,8 1235,7 |
6,57 6,78 6,87 7,09 7,83 8,06 8,56 9,38 9,67 |
0,85 0,90 0,98 1,0 1,3 1,4 1,6 1,8 2,0 |
|
MEA-PAA |
||||||
|
0,01 0,03 0,05 |
1,38 1,67 2,65 |
0,48 0,67 1,0 |
3,8 6,9 11,5 |
3,7 6,8 11,6 |
7,05 7,63 7,78 |
0,87 0,89 0,94 |
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
|
0,1 0,3 0,6 0,8 1,0 2,0 |
3,87 6,35 7,56 12,34 14.98 21,5 |
2,35 5,46 6,89 11,29 14,3 15,9 |
28,4 17,3 13,9 11,3 4,8 4,3 |
25,4 90,5 120,8 237,4 704.8 1125,3 |
7,90 8,09 8,92 9,47 9,93 9.98 |
1,2 1,4 1,7 1,9 2,0 2,1 |
|
PEA-PAA |
||||||
|
0,01 0,03 0,05 0,1 0,5 0,9 1,1 2,0 4,0 |
1,24 2,45 2,98 4,39 7,92 8,67 12,34 14,93 22,35 |
0,46 1,22 1,98 3,53 4,59 6,43 10,23 13,59 21,23 |
45 39,4 38,5 35,4 23,5 14,2 11,5 4,8 4,3 |
3,9 4,0 10,3 23,8 87,3 117,3 225,5 693,7 1130,6 |
8,03 8,39 8,93 9,37 9,98 10,38 11,45 12,29 13,76 |
0,79 0,83 0,89 0,92 1,2 1,4 1,6 1,8 2,0 |
In order to determine the intrinsic viscosity [η]c→0 the dependence of the reduced viscosity on the concentration is represented as the inverse relationship of the reduced viscosity([η]c→0) on the square root of the concentration (c), proposed by Fuossom-Strauss [2]. According to this relationship (Figure 1) of the straight lines extrapolation to the y-axis you can find the characteristic impedance of a single macromolecule in an infinitely dilute solution (with с→0), and gives an indication of the molecular weight of the polymer.
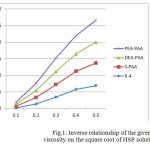 |
Figure 1: Inverse relationship of the given viscosity on the square root of HSP solutions Click here to View figure |
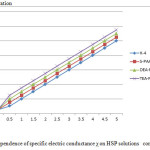 |
Figure 2: Dependence of specific electric conductance χ on HSP solutions concentration Click here to View figure |
When comparing the values of HSP [η]c→0 it can be noted that the intrinsic viscosity of the synthesized polymers is higher than that of K-4. In our view, this indicates an increase in the molecular weight of the “S-PAA” “MEA PAA” and “HP-PAA” by the interaction of K-4 macromolecules with the molecules of ethanolamine.
Polyelectrolyte character of the “M- PAA” series polymers synthesized by us has been confirmed by conductometric measurements. The curves on Figure 2 show that with the increasing concentration the specific conductivity of PE solutions increases. This is due to the fact that with the increasing concentration of PE the number of charged particles per volume unit increases.
The stability of the resulting polymers to oxidation and thermal effects were examined by the derivatograph. The heating rate is 65 dg/min, the temperature range is 293-870 K.
The stability of water-soluble synthetic polyelectrolytes when exposed to thermal oxidation can be judged by the results obtained by dynamic thermogravimetry (Table 2).
Table 2: Characteristics of the thermal stability of “S-PAA” “MEA PAA”, “HP-PAA” and K-4 polymers
| Polyelectrolyte | Т of exothermic effect, К | Е Activation energykJ / mol | Weight loss,% | Initial sample, 10-6 kg |
| S-PAA MEA-PAA HP-PAAK-4
|
706,5679,4
679,4 665,02 |
95,890,6
90,2 75,4 |
24,734,8
37,3 47,8 |
548,1578,9
548,2 549,9 |
Table 2 shows that weight loss during thermogravimetry K-4 is 48.2%, and the synthesized polymer S-PAA is 24.7% i.e. 1.5 times smaller. Weight loss of polymers MEA-PAA and HP-PAA is 34.8% and 37.3, respectively.
Exothermic reactions occur at different temperatures. For S-PAA, this value is 706.5 K, for the MEA-PAA it is 679.4 K and for HP-PAA it is 679.4 K.
The activation energy was calculated as the primary kinetic parameter for quantifying the process of thermal breakdown. The activation energies are shown in Table 3.
Table 3: The dependence of the rate constant (C) and exponent (n) on the S-PAA and MEA-PAA polymers concentration.
|
№ |
Polymer | Polymer concentration , % | Rate constant, C |
Exponent, n |
| 12
3 4 5 6 |
S-ПААS-ПАА
S-ПАА МEА-PАА МEА-PАА MEA-PАА |
0,010,05
0,10 0,01 0,05 0,10 |
1,141,45
2,06 0,68 1,24 1,67 |
0,890,73
0,36 0,47 0,39 0,24 |
According to the kinetic dependences by Veberreytor-Okubo method [3] rate constants of the adsorption layer formation K and the exponent n have been calculated. Under this method, the value of C is determined by the segment intercepted by the straight line dependence of lg τ from lg [2,3 lg (σ-σ͚) / σ-σ͚] on the vertical axis.
According to C values shown in Table 3, it can be noted that the value of C increases with the concentration, that is the access to the interface is easier.
From the values of n we can judge on what stage the adsorption process is rate-limiting: either the diffusion of macromolecules to the interface or the deformation of the macromolecules on this border. When n ~ 0,5 the adsorption process is determined by the diffusion of macromolecules to the interface, when n ~ 1 the conformational changes of macromolecules in the adsorption process are critical.
Figure 3 shows the dependence of the viscosity on pH, according to which the isoelectric point (IEP) of the synthesized polyelectrolytes is in the range of pH 2.7-3.0. At this point the viscosity of HSP solutions has a minimum value due to the fact that the macromolecules are in the most dense, convoluted conformation.
At pH lower than IEP more positive charges appear in the circuit. At low pH, the ionization of the acid groups is suppressed and polyampholyte assumes the character of poly base. At pH values above the IEP polyampholyte begins to show properties of poly acid.
Measurement of the water solutions of the synthesized polyelectrolytes surface tension allowed us to detect the decrease in the values of the surface tension with increasing concentration of the solution at the liquid-gas border. The ability to change the surface tension changes in the row: S-PAA> MEA-PAA> HP-PAA, which is confirmed by the calculation of the surface activity of the HM surfactant which equals 250 for S-PAA, 800 for MEA-PAA and 1900 for HP-PAA.
The adsorption increases in the row: S-PAA> MEA-PAA> HP-PAA. The calculation of the maximum value of adsorption at the interface liquid- gas showed the following values: for S-PAA G͚ =1·10-9, for the MEA-PAA G͚ = 0,05·10-9, for the HP-PAA G͚ = 0,03·10-9.
The size of HM surfactant macromolecules in the adsorption layer varies in the row: S-PAA> MEA-PAA> HP-PAA and is respectively 550 А02, 333 А02, 15 А02.
The thickness of the adsorption layer decreases from S-PAA to HP-PAA.
Adsorption characteristics indicate that the obtained series of water-soluble “MEA PAA” polymers possess both polyelectrolyte and surfactant features, which makes us expect their effect on disperse systems.
References
- Tager A.A. Physics and chemistry of polymers. Moscow.: Goschimizdat, 1992, .23-26
- Fuoss R, Strauss U.J. Polymer. Sci., 1948, 3.246,
- Febbereitor K.J. Temperature dependence of surface tension // Coll.and Polym. Sci., 1978. 256. (5), 490-493.

This work is licensed under a Creative Commons Attribution 4.0 International License.









