Structural Studies of Divalent Transition Metal Cobalt With Bis-(2,6-Diacetyl Pyridine Monoxime) 1,2-Di-Iminobenzene.
Shamshad Ahmad Khan* and Kumari Priti
Department of Chemistry, D. A. V. P. G. College Siwan (Bihar)
DOI : http://dx.doi.org/10.13005/ojc/310468
Article Received on :
Article Accepted on :
Article Published : 21 Oct 2015
The complexes of divalent Cobalt with bis(2,6-diacetyl pyridine monoxime)1,2-di-iminobenzene have been prepared separately in presence of respective salts. The general molecular formula of the complexes have been found to be [Co(L)]X2 where L=Bis(2,6-diacetyl pyridine monoxime)1,2-di-iminobenzene, X=chloride, bromide, sulphate, perchlorate, nitrate, acetate ions, On the characterisation of the complexes by usual physico-chemical method, all the complexes have been found to be monomeric and octahedral in geometry.
KEYWORDS:Co(II)complexes; octahedral; monomeric; condensation and Schiff base ligand
Download this article as:| Copy the following to cite this article: Khan S. A, Priti K. Structural Studies of Divalent Transition Metal Cobalt With Bis-(2,6-Diacetyl Pyridine Monoxime) 1,2-Di-Iminobenzene. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Khan S. A, Priti K. Structural Studies of Divalent Transition Metal Cobalt With Bis-(2,6-Diacetyl Pyridine Monoxime) 1,2-Di-Iminobenzene. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12032 |
Introduction
The co-ordination chemistry of Schiff base ligand is interesting because of their ability to form complexes with transition metal ions. In general transition metal ions and its complexes with Schiff base ligand have been studied widely in world due to their vast scope in the field of agriculture[1] and medicine[2,3]. There are numerous ligand containing nitrogen and oxygen donor atoms, out of which Schiff base of drug chemical is widely used as the ligand which contains oxygen donor atom in addition to the nitrogen atom of the azomethine (>c=o) group to form the stable complexes with the transition metals.
Here complexes of Co(II) with Schiff base derived from 4-hydrazino [3,2- d] benzofuro pyrimidine and 4-hydroxy-3-aldehydodiphenyl was reported by A.C. Hiremath[4] and co-workers. Patel and Patel[5] have reported the polychelates of Co(II) and have been prepared from polymeric Schiff base derived from 5-5’-methylenebis (3-bromo salicylaldehyde and hexane-1,6-diamine. Shaikh kabeer and his co-workers[6] reported that some new Schiff bases have been synthesised and tested for their antibacterial and antifungal activity. Mrs P.R.Shukla and his co-workers[7] reported complexes of Co(II) with Schiff bases derived from 2-pyrolidine and aromatic amines. A.Syamal and his co-workers[8] reported Co(II) complexes with Schiff bases derived from salicylaldehyde or substituted salicylaldehyde and ortho-aminobenzyl alcohol.Recently S.N Thakur and his co-workers[9-11] have reported the complexes of Co(II) with various ligands (β-hydrxy, α-Naphathalidine Anthranilino hydroximic acid, o-bis-(acetylacetonato) phenylene diamine, 3-(imino acetyl acetonato) propane-1-amine.
In this work the preparation of the Schiff base ligand, bis(2,6-diacetyl pyridine monoxime)1,2-di-iminobenzene was done by only two steps reaction and both the steps are condensation process. A number of complexes of Co(II) with ligands having nitrogen and oxygen as donor atoms are reported in which ligands behave as hexadented ligands.
Finally the preparation of cobalt(II) complexes with the Schiff base ligand bis(2,6-diacetyl pyridine monoxime)1,2-di-iminobenzene is also very easy work.
In conclusion, we have developed and synthesized very useful, absolutely clean and very high yielding, eco friendly Co(II) complexes.
Chemicals Required
2,6-diacetyl pyridine, hydroxylamine hydrochloride, O-phenylene diamine, Chloride/Bromide/nitrate/sulphate/perchlorate/acetateof Co(II) cation, organic solvent like acetone, alcohol etc.
Experimental
Preparation of the Schiff base ligand[12]
The Schiff base ligand bis-(2,6-diacetyl pyridine monoxime) 1,2-di-iminobenzene has been prepared in two steps. In first step, 3.5 grams (0.05 mole) of concentrated aqueous solutiom of hydroxylamine hydrochloride was added to an alcoholic solution of 60.5 grams (0.04) 2,6-diacetyl pyridine and cooled upto -50C. To this reaction mixture further added 20% aqueous solution of 12 grams (0.03 mole) NaOH dropwise with rapid stirring and temperature of the resultant mixture was maintained below 0oc. The colour of the solution became pink. After 90 minutes it was diluted with water and just acidified with glacial acetic acid. Allowed the mixture to stand for 30 minutes and then filtered through succession. The residue was recrystallised from aqueous alcohol (60 volume % alcohol). A white needle type crystal were obtained which was washed with water and dried. The melting point of this monoxime was found to be 218oC.The chemical reaction taking place during condensation process is given in figure-1.
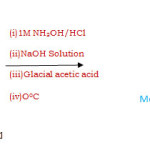 |
Figure 1 Click here to View figure |
In second steps, 1.08 grams (0.01 mole) 1,2-diaminobenzene was mixed with 2,6-diacetyl monoxime (0.02 mole) with the help of agate and mortar. They went into homogeneus viscous liquid after triturating them together for nearly two hours. The mortar was kept in refrigerator for overnight. The compound was dried under vaccum and recrystallised from a little of alcohol. The melting point was found to be 1840C. The compound was further analysed and found to contain C=67.11%, H=5.43% and N=19.26% respectively, Which corresponds to the molecular formula (C24H24N6O2). The chemical reaction taking place during condensation process is given in figure-2.
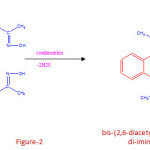 |
Figure 2 Click here to View figure |
Preparation of the Complexes
An alcoholic solution of 0.43 grams (0.001 mole) of the Schiff base ligand bis-(2,6-diacetyl pyridine monoxime) 1,2-di-iminobenzene was gradually added to an alcoholic solution of 0.24 grams (0.001 mole) of Cobalt (II) chloride hexahydrate.The molor ratio of metal and the ligand was always kept in 1:1 ratio respectively. The resulting solution was shaken well and refluxed on water bath about 45 minutes. On cooling the solution under tap water, dark brown precipitate was obtained which was separated by filtration. The precipitate was washed with alcohol, recrystallised and dried over KOH pellets placed in desiccators and found to contain Cobalt=10.40%, Carbon=51.36%, Hydrogen=4.32%, Nitrogen=15.20%, and Chlorine=20.76% respectively, which corresponds to the molecular formula [Co(C24H24N6O2)]Cl2.
The complexes with the ligand was prepared separately keeping the metal-ligand molar ratio as 1:1 in each case in presence of respective metal salts.
Table 1 : Analytical data of the complexes %: Found(calcd)
| Compounds | Metals | Carbon | Hydrogen | Nitrogen | Chlorine | Bromine | Sulphur |
| [Co(C24H24N6O2)]Cl2 | 10.56(10.40) | 51.62(51.36) | 4.30(4.32) | 15.05(15.20) | 12.72(12.76) | ||
| [Co(C24H24N6O2)]Br2 | 9.13(9.10) | 44.70(44.54) | 3.72(3.70) | 13.02(13.06) | 24.50(24.36) | ||
| [Co(C24H24N6O2)]So4 | 10.10(9.96) | 49.40(49.18) | 4.11(4.10) | 14.41(14.44) | 5.48(5.46) | ||
| [Co(C24H24N6O2)](Clo4)2 | 8.58(8.44) | 41.98(41.76) | 3.49(3.50) | 12.24(12.28) | 10.35(10.24) | ||
| [Co(C24H24N6O2)](No3)2 | 9.64(9.52) | 47.14(46.96) | 3.92(3.90) | 18.33(18.36) | |||
| [Co(C24H24N6O2)](Ac)2 | 10.13(9.96) | 53.70(53.53) | 5.16(5.20) | 14.46(14.50) |
Result and Discussion
The structure of the complexes are proposed by the interpretation of the result of elemental analysis, magnetic moment, electrical conductance, electronic spectra behaviour of the complexes and I.R. spectral nature of the ligand and the complexes under taken for the present investigation.
Magnetic Properties of Complexes
The magnetic moment of the complexes were measured by Gouy΄s method using the [ Hg(NCS)4] as calibrant. The value of magnetic moment of Co(II) complexes with the ligand obtained in the range of 4.84 to 5.02 B.M[13], which indicate octahedral arrangement of the donor atoms of the ligand molecule around the central metal cation.
Electrical Conductivity
The electrical conductivity of solution of complexes were measured by conductivity meter, bridge in DMF at 250c. Pure DMF and conductivity water were used as solvent . The value of electrical conductance of Cobalt(II) complexes with ligand obtained in the range of 152 to 164 ohm-1cm2mole-1 indicate electrolytic nature of the complexes.
Electronic Spectra
The electronic absorption spectra of the complexes were recorded with Hitachi-320 spectrophotometer.
In our present investigation two bands are obtained for the Cobalt(II) complexes. The first band obtained in the range of 14400-15200 cm-1 is broad and unsymmetrical due to J.T.distortion. The first transition takes place between 4t1g(F) → 4T2g(F) (4Eg+4B2g). The second band obtained in the range of 22250 to 23150 cm-1 is sharp and symmetrical due to 4T1g(F) → 4T1g(P) which is spin as well as symmetry allowed transition. The electronic transition spectra indicates octahedral nature of Cobalt(II) complexes.
Table 2: The colour, magnetic moment, electrical conductivity and electronic spectra data of complexes.
| Complexes | Colour | µeff(B.M.) | MagneticMoment | AmOhm-1cm2mole-1 | ν 1(cm-1) | ν 2(cm-1) | C.T.band |
| [Co(C24H24N6O2)]Cl2 | Dark brown | 5.02 | Paramagnetic | 154 | 15200 | 23100 | 33800 |
| [Co(C24H24N6O2)]Br2 | Yellowish brown | 4.96 | Paramagnetic | 160 | 14800 | 23000 | 34500 |
| [Co(C24H24N6O2)]So4 | Light brown | 4.90 | Paramagnetic | 164 | 14400 | 23150 | 34300 |
| [Co(C24H24N6O2)](Clo4)2 | Dark brown | 5.00 | Paramagnetic | 158 | 14900 | 22600 | 33900 |
| [Co(C24H24N6O2)](No3)3 | Dirty brown | 4.84 | Paramagnetic | 152 | 14600 | 22400 | 34100 |
| [Co(C24H24N6O2)](Ac)2 | Brown | 4.88 | Paramagnetic | 156 | 15000 | 22250 | 33700 |
I. R. Spectra
The Infra red spectra of complexes and ligand were recorded in nujol mull on Perkin Elemer 577 Spectrophotometer.
Table 3 : I. R. Spectra of the ligand and Co(II) complexes
| Ligand &the complexes | νO-H | νC=NAzomethine | νC=NOxime | νN-O | νPyridine | νN-OH |
| Ligand | 3310 | 1640 | 1460 | 1090 | 1580610410 | 1695 |
| [Co(C24H24N6O2)]Cl2 | 3315 | 1600 | 1490 | 1115 | 1605620425 | 1690 |
| [Co(C24H24N6O2)]Br2 | 3310 | 1600 | 1485 | 1120 | 1600625420 | 1700 |
| [Co(C24H24N6O2)]So4 | 3300 | 1590 | 1495 | 1110 | 1595625415 | 1705 |
| [Co(C24H24N6O2)](Clo4)2 | 3305 | 1595 | 1490 | 1120 | 1600625425 | 1720 |
| [Co(C24H24N6O2)](No3)3 | 3310 | 1585 | 1500 | 1115 | 1605620420 | 1710 |
| [Co(C24H24N6O2)](Ac)2 | 3300 | 1590 | 1495 | 1115 | 1595620415 | 1715 |
By The comparision of I.R. Spectral data of the ligand and the complexes, it has been found that there are appreciable changes in the frequency of νO-H, νC=N, νN-O, νN-OH indicating participation of these groups in the band formation with the metal cations.
The Schiff base ligand bis-(2,6-diacetyl pyridine monoxime) 1,2-di-iminobenzene displays a broad band at 3310 cm-1 in the ligand molecule.This band position obtained due to the vibration of (O-H) groups indicates the presence of H-bonding between two (N-O-H) groups present in the ligand molecule. There is no change in the band position after complex formation. This indicate that there is no deprotonation of groups remain intact in the complexes. This means that there is no participation of (OH) group in bond formation with metal cation.
A broad band obtained at 1460 cm-1 in the ligand molecule due to the vibration of (-C=N-) of oximino group. This band was shifted towards higher frequency side in the complexes. This band was shifted towards higher frequency side in the complexes. This increase in the frequency indicate that >C=N-O-H is present in the neutral form and nitrogen atoms of oximino group is coordinated with the metal ion. The further support of this arguement is obtained by the presence of a band obtained at 1695cm-1 in the ligand which corresponds to N-O-H scissoring. A broad band obtained at 1490cm-1 in the ligand molecule has been shifted to higher frequency by 30 to 40 cm-1 in the complexes. This increase in the frequency of (N-O) group indicates the participation of metal cation.
A broad and sharp band obtained in the ligand molecule at 1640 cm-1 due to the vibration of azomethin[14](>C=N) group has been reduced to 1580 cm-1 in the complexes. This reduction in the frequency of azomethine (>C=N) group indicates the bond formation with metal cation.
The appearance of spectrum of three more bands in the spectrum of ligand in the vicinity of (1570-1580), (600-610) & (400-415) is an evidence in support of the presence of pyridine ring[15-21]. These bands can be assigned to pyridine ring deformation, in plane deformation and out of plane ring deformation. . The upward shifting further indicates that nitrogen atom of pyridine ring has taken part in co-ordination with metal ion for the complex formation. The highest energy pyridine band 1570-1580 cm-1 corresponds to νC =N of the heterocyclic ring. A shift on high frequency side has indicated that there has been increase in the double bond character as a consequence of co-ordination with N-donor atom.
By the comparison of I.R. Spectral behaviour of the ligand and complexes, it is concluded that the ligand behaves as hexadentate molecule.
A sharp and medium band obtained in the range of 410 to 425 cm-1 in the complexes due to the vibration of (M-N) bond confirms the co-ordination of nitrogen atoms of different environment in the bond formation with the metal cations.Thus two aldimino nitrogen atom, two nitrogen atoms of pyridine ring and two oximino nitrogen atoms are the bonding sites of the ligand molecule.
The complexes of Co(II) cations with the ligand bis-(2,6-diacetyl pyridine monoxime) 1,2-di-iminobenzene are cationic complex their charges has been satisfied by the anions of respective metal salts used for the complex formation.
A pair of band obtained in the range of 2910 cm-1 to 2924 cm-1 and 1370 cm-1 to 1380 cm–indicates the presence of C-H and –CH3 group in the ligand as well as in the complexes. There are no band around 1740 cm-1 for >C=O group. The absence of this band in the complexes makes it clear that the carbonyl group of 2,6-diacetyl pyridine was completely replaced by >C=N- when allowed to react with O-phenylene diaminobenzene.
Thus on the basis of elemental analysis, value of electrical conductivity and magnetic moments and electronic spectra and interpretation of the I.R.Spectra of the ligand and the complexes, the probable structure of [Co(L)]X2 complexes are suggested to be tetragonally distorted octahedral in nature as shown in figure-3.
The structure of the complex of Co(II )is given below:
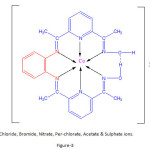 |
Figure 3 Click here to View figure |
Acknowledgement
The authors are thankful to Dr. B.K. Tiwari, Principal of D.A.V. PG COLLEGE SIWAN, J.P. University, Chapra for providing available library and laboratory facilities and cordial behaviour during the course of the whole research work.
Refrences
- Cyba,H.A., U.S.PAT Ze 3398170(1988) :Chem.Abstr., 69, (1968), 6363
- Snami,T. & Umezana, S. : Bull.Chem.Soc.Jpn., 29,(1956)875
- Theiss,H., Schoemberger,H. and Borah,K. : Arch.Pharm., 299(1966)1031
- Halli,M.B., Reddy,K.M., Qureshi,Z.S. & Hiremat,A.C. : Indian J.Chem., Vol.30A,(1991), 290-292
- Patel,V.J. & Patel,M.N : Indian J.Chem., 28A,(1989)434
- Shaikh Kabeer, M.A.Baseer and N.A.Mote: Asian J.Chem. 13(2001)496.
- Mrs. P.Shukla, Jayant Bhargava and Gopal Narain: J. Indian Chem.Soc., 60 (1983) 287
- A. Syamal and Bhuvnesh K. Gupta: J. Indian Chem.Soc., 58 (1981) 413
- S.N.Thakur et.al. : Asian J.Chem., 13(2001) 1001-1005
- S.N.Thakur et.al. : Asian J.Chem., 12(2000), 557-560
- S. N. Thakur et.al. : J. Chem.tracks., 13(2011), 581-584
- F. G. Mann and B. C. Saundars : Practical organic chemisty, 95-96
- R. S. Nyholm : J. C. S. 14 (1954)
- Belford, R.L., Calvin,M. & Belford, G : J. Chem. Phys., 1957, 26, 1165
- Pattel, Y.N. & Shahi,J.R.:Indian J.Chem. 1985, 24A, 800
- Goodgame,D.M. & Cotton,F.A.:Ibid, 1961, 2298
- J. H. Cooley et al.:J.O.C., 25, 1734(1960)
- H. E. Baumgarsen et al.:J.O.C., 30,1203(1965)
- N. B. Colthup, L.H.Dally and S. E. Wiberly: Introduction to Infrared and Raman Spectroscopy, Academic Press, New York (1964)
- C. N. R. Rao: Chemical applications of infrared spectroscopy, Academic Press, New York (1963)
- K. Nakamoto:Infrared spectra of co-ordination

This work is licensed under a Creative Commons Attribution 4.0 International License.









