Oxidative Desulfurization of kerosene in the presence of iron chlorideionic liquid catalyst and ultrasound waves
Maryam Sadat Seyedi1, Manouchehr Bahmaei1* and Amir Farshi2
1Department of Chemistry, Islamic Azad University, North Tehran Branch, Tehran, Iran 2Refining Technology Development Department, Research institute of petroleum industry (RIPI)
DOI : http://dx.doi.org/10.13005/ojc/310472
Article Received on :
Article Accepted on :
Article Published : 02 Dec 2015
Oxidative Desulfurization of kerosene refinery in Tehran with sulfur content of 0/293% with iron chloride - hydrogen peroxide and ultrasonic liquid catalysts in the presence of acetic acid - formic acid and an oxidizinghydrogen peroxide has been studied. The effects of operating parameters such as temperature, reaction time, mole ratio of moles of sulfur oxidation (no/ ns),mole ratio of moles of acid per mol of sulfur (nacid/ ns (on the desulfurization of kerosene checked(the molar ratio of oxidant to 15-40 and 20-80 mole ratio of sulfur to sulfur acid)The results showed that the optimal conditions for the removal of sulfur from iron chloride catalyst system kerosene by 93% and sulfur content of residual 128 ppm is obtained. The effect of ultrasonic waves on system performance oxidationwas studied, the results showed that the percentage of desulfurization systems, oxidation of acetic acid- hydrogen peroxide in combination with ultrasound (96%) and without ultrasound was 93%, which indicates improved performance oxidation The presence of ultrasound.
KEYWORDS:Oxidative Desulfurization of kerosene; ultrasound; ironchloride; Hydrogen Peroxide
Download this article as:| Copy the following to cite this article: Seyedi M. S, Bahmaei M, Farshi A. Oxidative Desulfurization of kerosene in the presence of iron chlorideionic liquid catalyst and ultrasound waves. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Seyedi M. S, Bahmaei M, Farshi A. Oxidative Desulfurization of kerosene in the presence of iron chlorideionic liquid catalyst and ultrasound waves. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12797 |
Introduction
New environmental concerns necessitate low sulfur requirements in hydrocarbon based distillate fuels so that desulfurization of petroleum-derived fuels becomes an important part of refining processes. fuels so that desulfurization of petroleum-derived fuels becomes an important part of refining processes. The sulfur-containing compounds not only cause an adverse effect on the quality of petroleum products by decreasing the American Petroleum Institute(API) gravity, but also are converted to SOx during combustion, being a major source of acid rain and air pollution.Therefore, nowadays, very stringent legislation for ultra-low-sulfur fuels are being imposed on oil refineries throughout the world to reduce the sulfur content of their products to a very low limit around 0.001% -0.002% [1].
At present, the conventional industrial process for removing sulfur-containing compounds from middle distillate fuels is hydrodesulfurization (HDS). To meet new sulfur standards with HDS process, operation at higher temperature and higher pressure with more active catalysts is indispensable, leading to higher investment and operating cost. Therefore, several new processes have been developed to remove these refractory sulfur-containing compounds satisfactorily. Such alternative processes include selective adsorption, biodesulfurization, oxidative desulfurization(ODS), etc [2].
Among these alternative processes, the ODS process has received much attention as an alternative process for the desulfurization because of its two main advantages relative to the HDS process[3]. The greatest advantage of ODS is its capability to carry out in the liquid phase under very mild temperature and pressure conditions. Another important feature of ODS is that the most refractory sulfur-containing compounds, which have less reactivity toward the HDS processs how high reactivity toward the ODS process[4]. Besides, the ODS process is a non-hydrogen consuming desulfurization method and can be applied whenever enough hydrogen sources are not available. This method of removing sulfur compounds is high and the sulfur-containing compounds in the form of thiophene and sulfide sulfur contained in the oil are removed with this method [5]. Liquid-liquid extraction enjoys a favoured position among the separation techniques[6].
The chromium-based reagents are widely used and powerful oxidation reagents[7].Thus, a desulfurization process based on this oxidation system has been developed and reported by Unipure Company in 2005[4].
Goth and Diaz separated sulfur and nitrogen from oil derivatives using nitrogen dioxide with methanol and managed to remove 66-87% of sulfur from diesel fuel[8,9].
Kim et al. presented a mechanism for oxidation Benzothiophene in the presence of ultrasound in 2003[10]
Durateet al.The effect of temperature on the reaction by ultrasound at 20 to 90° Cwereevaluated. Durate reaction time for the oxidation of sulfur compounds and colleagues have evaluated[11].
In this study, performance of different advanced oxidation systems (acetic acid-hydrogen peroxide, formic acid-hydrogen peroxide, iron chloride-hydrogen peroxide, iron chloride-hydrogen peroxide and acetic acid-hydrogen peroxide and ultrasound) is done for desulfurization of Iran’s refining kerosene (Tehran Refinery) with initial sulfur 2930 ppmw and extraction acetonitrile and optimization various operating parameters such as temperature and time of reaction, molar ratio of oxide to sulfur and molar ratio of acid to sulfur is investigated, and the optimum value for the ratio of sulfuroxide and sulfur molar ratio of acid to 25 and 60 respectively.as well as ultrasonic performance on extraction oxidized sulfur compounds has been studied.
Experimental
Materials and Method
All used chemicals in this study including 98% acetic acid, formic acid 99 %, aceto-nitrile 99.9% and hydrogen peroxide with purity 30 % and iron chloride (ш) 6 Abe have been prepared in Merck of Germany without any purification and kerosene with sulfur content of 2930 ppmw is obtained from refining company in Iran (Tehran Refinery).
Analytical method
Sulfur analysis device with radiation X-ray fluorescence that its detection range is from 0.001 – 0.0005 w was used to determine the total content of sulfur in kerosene. The total sulfur analyzer (X-ray fluorescence SLFA Horiba 2800) is made in Japan and analysis sulfur in oil cut according America standard (ASTM-D-4294). This device consider water and metal ions as sulfur so,oil samples should be removed to measure sulfur of above materials.A Renee nickel machine that is based on standard (ASTM) is used to measurement sulfur in samples containing iron ions.
The Experimental Part of Tests
Method of Oxidation Test
In any test method, 100 ml of Kerosene feed of purification unit HDS with sulfur content of 2930ppmw
is transferred to glass reactor equipped with a thermometer and reactor is placed in warm water bath at a constant temperature then appropriate amount of catalyst (solid or liquid) was added to reactor and mixed is mixture and heated continuously to reaches desired temperature of reaction After this time, appropriate amount of hydrogen peroxide is added to reaction mixture and this point is considered as zero point of reaction. The time of this reaction has been 90 minutes.
Test Method for Extraction Sulfones from kerosene
The hydrocarbon phase from reaction is extracted during two times with acetonitrile, water (with a volume ratio of 50%). Extracting operations is as follow that an appropriate amount of aqueous solvent (Acetonitrile and water) and organic solvents (Hydrocarbon phase) are transferred to a separator funnel and are shaken in temperature of funnel environment for 10 to 15 minutes. So, it consists of two phases that lower phase is (water phase) and upper phase is (hydrocarbon phase). Kerosene samples were sent to laboratory of the Institute of Petroleum Industry in order to determination sulfur content by X-ray fluorescence device.
Results and Discussion
Catalytic Effect of Iron Chloride
The effect of iron chloride catalyst in desulfurization plays its role by extraction sulfur compounds to water phase and change to sulfones and sulfoxides in low temperature and low weight. The experiment was conducted in 100 ml of fuel with hydrogen peroxide in the presence of 50 ml to 0 /05 g catalyst under different temperatures. The results of the experiment are presented in Figure 1.
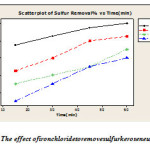 |
Figure 1: The effect of iron chloride to remove sulfur kerosene using ODS Click here to View figure |
[The system of hydrogen peroxide – iron chloride: at different temperatures 25 ° C to 60 ° C with a sulfur content of 2930 ppmw and 50 ml of hydrogen peroxide and 0/05 g of iron chloride oxidation tested in 100 ml of kerosene the is.Extraction conditions: temperature 25 ° C and 50% by volume acetonitrile solvent – water and oil than double the amount of fuel twice during extraction ].
The results of tests using small amounts of iron chloride catalyst(which is solublein the aqueous phase) is significantly reduced sulfur kerosene. It makes use of large amounts of removal of oxidation catalysts desulphurization reaction liquid kerosene which is using them can lead to a change in the use of these catalysts in the reaction of ODS.
The Effect of Ultrasound
Desulphurisation increase the efficiency of ultrasound in less time and with ultrasound – mechanical stirrer and ultrasound alone 97% and 96%, respectively, has been able to remove. Desulphurisation efficiency in the presence of ultrasound increased 2/83 percent compared to the absence of ultrasound has shown that the test results are shown in Figure 2.
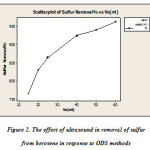 |
Figure 2: The effect of ultrasound in removal of sulfur from kerosene in response to ODS methods |
[Systemacetic acid- hydrogen peroxide: at a temperature of 25 ° C with a sulfur content of 2930 ppmw and 100 ml fuel with 70 ml of acetic acid and hydrogen peroxide in the presence of various volumes of 15 to 60 ml at 30 min of reaction conditions extraction temperature of 25 ° C and 50% by volume solution of distilled acetonitrile solvent – water and oil than double the amount of fuel twice during extraction].
The experiment was carried out at 25 ° C, 100 ml of fuel with 70 ml of acetic acid and hydrogen peroxide in the presence of various volumes of 15 to 60 ml at 60 minutes of the reaction was a combination of ultrasound waves and mechanical stirrer that test results in Figure 3 is presented.
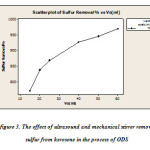 |
Figure 3: The effect of ultrasound and mechanical stirrer remove sulfur from kerosene in the process of ODS Click here to View figure |
[Systemacetic acid- hydrogen peroxide: at a temperature of 25 ° C with a sulfur content of 293/0% 100 ml fuel with 70 ml of acetic acid and hydrogen peroxide in the presence of various volumes of 15 to 60 ml at 30 min of reaction conditions extraction temperature of 25 ° C and 50% by volume solution of distilled acetonitrile solvent – water and oil than double the amount of fuel twice during extraction].
Conclusions
Desulfurization of kerosene oxidative systems using iron chloride – hydrogen peroxide and acetic acid -hydrogen peroxide and various operating parameters such as ultrasound study and the effect of temperature and reaction time, the effects of the molar ratios of no / ns, nacid / ns were investigated. The testing was done following results were obtained:
Desulfurization with increasing temperature due to increased reaction speed.
The removal of sulfur compounds from kerosene initial reaction was performed in 30 minutes.
Using solid catalysts ferric Fe3 + in a short time and with low metal ion concentration accelerates the oxidation is Desulfurization.
Enhanced ultrasound causes a significant increase in the percentage of sulfur-containing compounds of kerosene have been removed.
The impact of oxidation on alone without extraction were studied and the results showed that oxidation to remove sulfur from kerosene Desulfurization alone have a significant impact on not only the form of the corresponding sulfur compounds to sulfones andsulfoxides. The characterization of kerosene before and after Desulfurization Oxidative Desulfurization that could adversely affect the properties of kerosene does, and this is a good way to remove sulfur compounds from kerosene.Most Desulfurization In this study, 97% removal of sulfur with oxidation of acetic acid-hydrogen peroxide with 70 ml of acetic acid and 60 ml of hydrogen peroxide with 100 ml of kerosene with a sulfur content of 2930ppmw in the presence of mechanical stirrer and ultrasound viewing is.
References
- Gore, W.,Method of desulfurization of hydrocarbons, U.S. Pat., 6160193(2000).
- Ma.X, Song.C, Appl. Catal., B. 2003,41, 207-238.
- Marafi .A, Rana.M.S, Stanislaus.A, A. Catal. Today.2010,153, 1-68.
- Kumar .A, Tuil.D, Non-conventional technologies for fuel desulphurization”19thworld petroleum congress, Spain 2008.
- Giavanovich. V.I, Lazorko.O.I, Pariv. P.M, Pysh, ev. S.V, Chem. Technol. Fuels Oils. 2006, 42(3), 159-166.
- Swamidoss C.M., Malkhede, D.D. Orient J Chem. 2010, 26(1), 117-122.
- Sonawane,V.Y, Hilage.N.P. Orient J Chem. 25(2), 2009, 25 (2), 307-312.
- Arledge. K.W, Brienza.A.R, Guth.E.D, Helgeson.N.L, Petroleum oil desulfurization process, U.S. Pat., 1975.3,919,402
- Chen. B, Haung. C, Li.Y, Liu. Z, Zhang.J, Energy Fuels. 2004, 46, 111–150.
- Chin .H.P, Jea. L.K, Kim .K.I, Seok. Y.J, ,Korean J. Chem. Eng. 2003, 20(6), 1045-1053.
- Bizzi. C, Duart .F, Mello. P, Fuel. 2011 90, 2158-2164.

This work is licensed under a Creative Commons Attribution 4.0 International License.









