Mechanistic and full Kinetic Study of the reaction between 4-chlorobenzaldehyde, malononitrile and dimedone using caffeine as a green catalyst
Halimeh Yaghoubian, Sayyed Mostafa Habibi-Khorassani* and Ali Ebrahimi
Department of Chemistry, Faculty of Science, University of Sistan and Baluchestan, P O Box 98135-674, Zahedan, Iran Corresponding Author E-mail: smhabibikhorassani@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/310431
Article Received on :
Article Accepted on :
Article Published : 04 Dec 2015
This article was conducted to investigate spectrophotometrically the kinetic and mechanism of the reaction synthesis of tetrahydrobenzo[b] pyrans via one-pot, three-component condensation of malononitrile, dimedone and 4-chlorobenzaldehyde in the presence of caffeine, as a new green catalyst. The effect of various parameters such as temperature, solvent, concentration and catalyst was determined. Pursuant to obtained data the reaction rate was obeyed second order kinetics and automatically calculated rate constants were in agreement with the Arrhenius and Eyring equations. From these the activation parameters (Ea, ΔG‡, ΔS‡, and ΔH‡) were determined. Also, in a mixture of water: ethanol (2:1), the rate constant was increased with respect to ethanol solvent that has lower dielectric constant. Furthermore, experimental data and steady state approximation corroborated the proposed mechanism. The results showed that the first step of the reaction mechanism is a rate determining step (RDS).
KEYWORDS:Kinetics; Mechanism; Tetrahydrobenzo[b]pyran; spectrophotometry technique
Download this article as:| Copy the following to cite this article: Yaghoubian H, Habibi-Khorassani S. M, Ebrahimi A. Mechanistic and full Kinetic Study of the reaction between 4-chlorobenzaldehyde, malononitrile and dimedone using caffeine as a green catalyst. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Yaghoubian H, Habibi-Khorassani S. M, Ebrahimi A. Mechanistic and full Kinetic Study of the reaction between 4-chlorobenzaldehyde, malononitrile and dimedone using caffeine as a green catalyst. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12920 |
Introduction
With increment environmental tendencies and regulative restriction encountered in pharmacological and chemical industries, extension of environmentally friendly benignant organic reactions has become decisive and wishing research. So, very of organochemists efforts are allocated into ‘green chemistry’, that means the solvents, catalysts and reactants are environmentally amicable. One-pot multi-component reactions illustrate a conceivable appliance to accomplish a nearby idealized synthesis due to performing impressive combination of three or more ingredients in a single process and eluding the use of abundant quantities of organic solvents, poisonous reagents, costly purification methods, also, possessing high efficiency of products and saving the energy[1]. One pot, three-component synthesis of tetrahydrobenzo[b]pyrans is one example of an MCR. Benzopyrans form the backbone of many natural products which represent an immense series of pharmaceutical activities including diuretic, antitumor, anticoagulant, antibacterial, spasmolytic, antiallergic, and potassium channel activators [2]. Some 2-amino-4H-pyrans can be utilized as pigments, photoactive materials, and potential biodegradable agrochemicals [3]. A variety of methods for synthesis of these compounds using a number of catalysts under ultrasonic or microwave irradiations or in an electrocatalytic system have been reported [4-7]. Serious problems such as multi-step reactions, high temperature conditions, costly catalysts or reagents, prolonged times of reaction; intense work-up, poor yields, issuant pollution, using specific apparatus, toxic solvents and etc. have restricted applying of these methods [8, 9]. Newly, green catalysts along with safe and inexpensive solvents such as water and mixture of water and ethanol, instead of detrimental solvents, have attracted much attention [10].synthesis of tetrahydrobenzo[b]pyrans 4, from aromatic aldehydes, malonitril 2, and dimedone 3, in the presence of varies catalysts was studied before [11]. In continuance of our recent works in kinetic investigations of new organic synthesis [12-22], we herein study experimental kinetics of this reaction from 4-chlorobenzaldehyde 1, in the presence of caffeine as a green and convenient available catalyst in a mixture of water: ethanol as a solvent (scheme 2). Reaction kinetics studies can constitute the convenient reaction mechanism as well as leading to optimizing the proceeding conditions of organic synthesis, chemical manufactures and analytical reactions.
 |
Scheme 1 |
Experimental Cection
4-chlorobenzaldehyde 1, malonitril 2, dimedone 3, caffeine and ethanol were obtained from Merck (Darmstadt, Germany) and Fluka (Buchs, Switzerland), and used without any purification. All of the experiments were done by a carry UV-Vis spectrophotometer model Bio-300 with a 1 mm light-path quartz cell.
General Procedure
At first, 10-2 M solution of compounds 1, 2, 3 and catalyst was prepared in a mixture of 2:1 water: ethanol as a solvent, separately and then, single spectrum of each compound was recorded at wavelength range 200-800 nm. In the second experiment, 0.2 ml of the solution of catalyst and reactant 2, 3 was added into the cell, respectively (because there is no reaction between them); then, 0.2 ml of reactant 2 was added into the mixture according to stochiometry of each compound in the overall reaction and progress of reaction was monitored at the ambient temperature (Fig 1). Accordingly, the appropriate wavelength at which reactants1, 2, 3 and catalyst have relatively no absorbance was found to be 370 nm; so, full kinetics and mechanism of the reaction was investigated at this wavelength. Hereupon, in all the experiments, the UV-Vis spectrum of the product was measured over the concentration range (10-3 M < M product <10-2 M) to demonstrate a linear relationship between the absorbance and concentration values. In the third experiment under same condition with the previous experiment the absorbance curve was recorded versus time at 25℃ and 370 nm (Fig. 2). Zero, first or second curve fittings can be drawn using the software associated with the UV-Vis instrument; also, the pertaining rate constants can be calculated automatically [23]. As can be seen in Fig. 2 the experimental absorbance data (dotted line) accurately fitted to second order curve (solid line); so, overall order of reaction in accordance with rate law, indicated in equation(1), can be written as: α + β + γ = 2
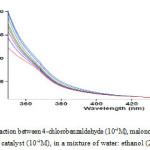 |
Figure 1: UV/Vis spectrum of the reaction between 4-chlorobenzaldehyde (10-2M), malononitrile (10-2M) and dimedone (10-2M) in the presence of caffeine catalyst (10-2M), in a mixture of water: ethanol (2:1) Click here to View figure |
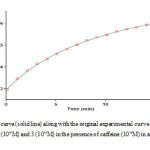 |
Figure 2: Pseudo second order fit curve (solid line) along with the original experimental curve (dotted line) for the reaction between reactants 1 (10-2M), 2 (10-2M) and 3 (10-2M) in the presence of caffeine (10-2M) in a mixture of water: ethanol (2:1) at 370nm Click here to View figure |
Results and Discussion
Effect of Solvent and Temperature
Several experiments were performed at various temperatures and solvents under the same concentration of each component (10-2M). The relevant rate constants obtained from software were listed in Table 1. The dependency of the rate constants (Lnkobs and Lnkobs/T) on temperature display compatibility with Eyring and Arrhenius equations. Moreover, the linearized form of Eyring equations was examined to compare these two cases with to gather. [16] (Figs. 3, 4). Activation parameters were given in Table 2.
Table 1: Values of observed rate constants for the reaction between 1, 2 and 3 in the presence caffeine catalyst at 370 nm
|
kobs(M.min-1) |
||||||||
| l(nm) | Solvent | (a) 45.0 ºC | (a) 40.0 ºC | (a) 35.0 ºC | (a) 30.0 ºC | (a) 25.0 ºC | (a) 20.0 ºC | (b) 40.0ºC |
| 370370 | Water: ethanol(2:1)ethanol | 10.431.23 | 8.19 | 6.68 | 5.39 | 4.22 | 3.31 | 8.33 |
a: 1 (10-2 M), 2 (10-2 M), 3 (10-2 M), catalyst (10-2M)
b: 1 (10-2 M), 2 (10-2 M), 3 (5×10-3M), catalyst (10-2M)
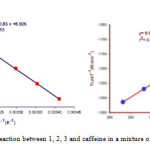 |
Figure 3: Eyring plots for the reaction between 1, 2, 3 and caffeine in a mixture of water: ethanol (2:1) at 370nm |
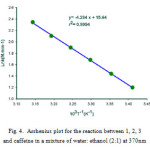 |
Figure 4: Arrhenius plot for the reaction between 1, 2, 3 and caffeine in a mixture of water: ethanol (2:1) at 370nm Click here to View figure |
Table 2: Activation parameters for the reaction between 1, 2 and 3 in the presence of caffeine catalyst at 370nm
a: according to Eyring equation, b: according to linearizied form of Eyring equation
|
solvent |
∆H‡(kJ.mol-1) | ∆S‡(J.mol-1.K-1) | ∆G‡(kJ.mol-1) |
Ea(kJ.mol-1) |
| Water: ethanol(2:1) | (a) 32.67(b) 32.68 | (a) -123.33(b) -123.32 | (a) 69.45(b) 69.45 | 35.20 |
The positive value of ∆H‡ (Table2) indicates that the reaction is endothermic and large negative value of ∆S‡ determines a transition state that is forcefully ordered than the reactants.
Also as can be seen from Table1, the reaction rate increases whit increasing temperature. Furthermore, in a solvent whit higher dielectric constant (mixture 2:1 water: ethanol) in comparison with lower dielectric constant (ethanol) the reaction rate is accelerated. That can be ascribed to different stability of activated complexes and reactants in these environments.
Effect of Concentration
In order to determine partial order with respect to compounds 1, 2 and 3 three experiments were perform in which the concentration of one of these components was selected much less (5×10-3) than the others (10-2) independently. In other words, pseudo-order conditions were established for these reactions. The absorbance curves were shown in Figs. (5-7).
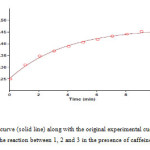 |
Figure 5: Pseudo first order fit curve (solid line) along with the original experimental curve (dotted line) related to 4-chlorobenzaldehyde 1, for the reaction between 1, 2 and 3 in the presence of caffeine at 370nm Click here to View figure |
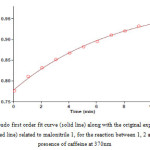 |
Figure 6: Pseudo first order fit curve (solid line) along with the original experimental curve (dotted line) related to malonitrile 1, for the reaction between 1, 2 and 3 in the presence of caffeine at 370nm Click here to View figure |
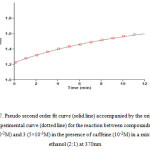 |
Figure 7: Pseudo second order fit curve (solid line) accompanied by the original experimental curve (dotted line) for the reaction between compounds 1 (10-2M), 2 (10-2M) and 3 (5×10-3M) in the presence of caffeine (10-2M) in a mixture of water: ethanol (2:1) at 370nm Click here to View figure |
As can be seen, under pseudo-order conditions, the reaction are first order whit respect to compounds 1 and 2 (Figs. 5, 6); in the other hands, α=β=1. Also by accordance of experimental data whit a second order fitting (Fig. 7) and as respects the calculated rate constants from this plot were similar to rate constants calculated from Fig. 2, we thus say that kobs are independent of concentration of compound 3. That is, the reaction order with respect to this compound is zero. So we can modify equation 1 as fallow:
Rate = kovr[1][2][cat], kobs = kovr[cate] (1)
Rate = kobs[1][2] (2)
Effect of catalyst
The reaction between 1, 2 and 3 was accomplished in the presence of agar as a second catalyst in a mixture of water: ethanol (2:1). The reaction rate was increased in comparison with the caffeine (Table 3); That is, agar has poor interaction with polar solvent compared to caffeine, so, it can have more liberty for accomplishment its catalytic role.
Table 3: Effect of Various Catalysts on the Reaction between 1, 2 and 3 at 45ºC and 370nm
| solvent | catalyst | kobs(M.min-1) |
| Water: ethanol(2:1) | caffeineagar | 10.4313.09 |
Proposed Mechanism
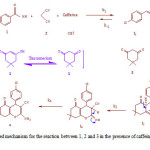 |
Scheme 2: The proposed mechanism for the reaction between 1, 2 and 3 in the presence of caffeine in a mixture of water: ethanol (2:1) |
 |
Scheme 3: a simplified stepwise scheme for the proposed reaction mechanism. |
To determine which step of proposed mechanism is rate-determining step, the rate law was written for final step of reaction:
Rate = k4[I3] (3)
Using the steady state approximation to obtain the concentration of all intermediates (I1, I2 and I3), Rate equation can be written as follows:

This equation is independent of rate constants for the third and fourth steps. Thus, these steps have no opportunity to be a rate determining step; Furthermore if k2[3]>>k-1[H2O] ; then, we can infer:
Rate = k1[1][2][cat] (6)
By comparison the equations 2 and 6, we can write:
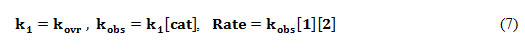
According to this equation, the overall order of the reaction is two and the reaction order with respect to compounds (1, 2and 3) is 1, 1 and zero, respectively; which was previously confirmed by the experimental data.
The presence of k1 in the rate law (equation 6) demonstrates that first step is a rate-determining step and step 2 should be a fast step. In this case, the transition state (Scheme 3, step 1) carries a dispersed charge; hereupon, solvent with higher dielectric constant can be more effectual on this dispersed charge compared whit reactants 1 and 2, that do not have any charge. Thus, the solvent stabilizes the species at the transition state more than the reactants. Therefore, Ea would have lower amounts, and increases the reaction rate.
Conclusion
Full kinetic and a mechanistic investigation of aforesaid reaction was undertaken using UV spectrophotometry technique. The results can be listed as follow:
- The reaction rate followed second-order kinetics so that the partial order of reaction toward 4-chlorobenzaldehyde, malononitrile and dimedone was respectively one, one and zero.
- In solvents with higher dielectric constant the rate of reaction increased and this can be pertained to different stabilization of reactants and activated complex in transition state.
- According to experimental data, the first step (k1) of proposed mechanism was identified as a rate-determining step and this was affirmed with steady-state approximation.
- Activation energy and parameters ΔG‡, ΔS‡ and ΔH‡ were calculated for the reaction.
- The reaction rate accelerated at higher temperatures.
Acknowledgments
We are grateful of Research Council of Sistan and Baluchestan University for the partial support of this research.
References
- Bandgar BS., Bandgar B P, Korbad BL., Totre JV, Patil S, Aust J Chem, 2007.,60., 305
- Sabitha G, Arundhathi K, Sudhakar K, Sastry BS, Yadav J S, Synth Communications, 2009., 39 .,433
- Ying-Lei Wang, Zhuo Li, Jun Luo, Zu-Liang Liu, J Chin Chem Soc, 2013.,60.,1431
- Tabatabaeian K, Heidari H, Mamaghani M, Mahmoodi NO, Appl Organometal Chem, 2012., 1., 1866
- Fotouhi L, Heravi MM, Fatehi A, Bakhtiari Kh, Tetrahedron Letters 2007., 48
- Jia Zheng, Yi-Qun Li, Arch Appl SciRes, 2011.,3.,381
- Naglaa M. Abd El-Rahman, Rita M. Borik, World Appl Sci J, 2014., 31 .,01
- Noori Sadeh F, Maghsoodlou MT, Hazeri N, Kangani M, Res Chem Intermed, 2014.,1.,1
- Habibi-Khorassani SM, Maghsoodlou MT, Ebrahimi A, Zakarianejad M, Mohammadzadeh P, Shahraki M. Oriental Journal of Chemistry 2008., 24(1) 73-82.
- Habibi-Khorassani SM, Maghsoodlou MT, Aghdaei E, Shahraki M, Prog React Kinet Mech 2012.,37.,301
- Shahraki M, Habibi-Khorassani S M; Dehdab M. RSC Adv, 2015.,5.,52508-52515.
- Habibi-Khorassani, SM., Maghsoodlou, MT., Shahraki, M., Hashemi-Shahri, Marjan., Aboonajmi J., Zarei, T. Iranian Journal of Catalysis 2014 4(4):241-246.
- Pourpanah S. Sh., Habibi-Khorassani S. M., Shahraki M. Chinese Journal of Catalysis 2015 36: 757.
- Dehdab M, Habibi-Khorassani SM, Shahraki M, Catalysis Letters, 2014.,144.,1790
- Shaharaki M, Habibi-Khorassani SM, Ebrahimi A, Maghsoodlou MT, Paknahad A, Prog React Kinet Mech, 2012.,37.,321
- Habibi-Khorassani S. M., Ebrahimi A., Maghsoodlou M. T., Asheri O., Shahraki M., Akbarzadeh N., Ghalandarzehi Y. Int J Chem Kinet. 2013.,45:596.
- Habibi-Khorassani S. M., Ebrahimi A., Maghsoodlou M. T., Shahraki M., Price D., Analyst. 2011., 136:1713.
- Shahraki M.,Habibi-Khorassani S. M., J Phys Org Chem. 2015 doi: 10.1002/poc.3424
- HabibiKhorassani SM, Maghsoodlou MT, Ghasempour H, Zakarianejad M, Nassiri M, Ghahghaie Z, J Chem Sci, 2013., 125.,387
- Zakarianejad M, Ghasempour H, Habibi-Khorassani SM, Maghsoodlou MT, Makiabadi B, Nassiri M, Ghahghayi Z, Abedi A, Arkivoc, iv(2013) 17
- Habibi-Khorassani SM, Maghsoodlou MT, Shahraki M, Talaie Far S, Mousavi MR, Adv in Phys Chem 2014.,1

This work is licensed under a Creative Commons Attribution 4.0 International License.









