Green corrosion inhibitors for carbon steel by green leafy vegetables extracts in 1 M HCl
Ghadah M. Al-Senani, Sameerah I. Al-Saeedi, Rasmiah Almufarij
Department of Chemistry, College of Science, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. Corresponding Author Email: gmalsnany@pnu.edu.sa
DOI : http://dx.doi.org/10.13005/ojc/310428
Article Received on :
Article Accepted on :
Article Published : 10 Nov 2015
The effect of some Green Leafy Vegetables (GLV) extracts namely Lactuca sativa (Lactuca), Eruca Sativa (Arugula), Petroselinum crispum (Parsley), and Anethum Graveolens (Dill) were investigated on inhibition of corrosion carbon steel in 1 M HCl solution using gravimetric (weight loss) method. The inhibition efficiency has increased as concentration of the extract increased. The inhibition efficiency has decreased as the temperature increased. The results obtained showed that GLV extracts inhibited the corrosion process by a physical adsorption mechanism that followed the Langmuir, Freundlich, and Temkin adsorption isotherm models. The adsorption thermodynamic parameters that were calculated include, free energy of adsorption (∆G°ads), activation energy (Ea), enthalpy of adsorption (ΔH°ads), and entropy of adsorption (ΔS°ads) are proposed for the corrosion of carbon steel in 1 M HCl in the absence and presence of GLV extract.
KEYWORDS:Carbon steel; Hydrochloric acid; Corrosion inhibition; Green Leafy Vegetables; Adsorption
Download this article as:| Copy the following to cite this article: Al-Senani G. M, Al-Saeedi S. I, Almufarij R. Green corrosion inhibitors for carbon steel by green leafy vegetables extracts in 1 M HCl. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Al-Senani G. M, Al-Saeedi S. I, Almufarij R. Green corrosion inhibitors for carbon steel by green leafy vegetables extracts in 1 M HCl. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12547 |
Introduction
Carbon steel is widely used in industries due to its good mechanical property. Carbon steel has been extensively used under different conditions in chemical and allied industries in presence of alkaline, acid and salt solutions. The investigation of corrosion of carbon steel is always a subject of high theoretical as well as practical interest. Chloride, sulphate and nitrate ions in aqueous media are particularly aggressive and accelerate corrosion. One way of protecting steel from corrosion is to use corrosion inhibitors. The known dangerous effects of most synthetic corrosion inhibitors are the motivation for the use of some natural products. The recent trend is towards environmentally friendly inhibitors. Most of the natural products are non-toxic, biodegradable and readily available in plenty. Several investigations have been reported using such naturally occurring substances as corrosion inhibitors for several metals in different media.
Recently, plant extracts have again become important as an environmentally acceptable, readily available, and renewable source for a wide range of needed inhibitors. Plant extracts are viewed as an incredibly rich source of natural chemical compounds that can be extracted by simple procedures with low-cost, and so the study of using plant extracts as corrosion inhibitors is an important scientific research field due to both economic and environmental benefits [1].
There are several reviews on the use of plant extracts as corrosion inhibitors. Recently aqueous extract of Banana peel [2]. The inhibitive action of Fruit peel aqueous extract [3], eco-friendly inhibitor L-Valine-Zn2+ [4], Eucalyptus Camaldulenis leaves extract [5], Ethanol extract of Fagus Sylvatica leaves [6], Gnetum Africana leaves extract [7], Osmanthus Fragran leaves extract [8], Seed extract of Psidium guajava [9], aqueous extract of Propolis [10], Juniper Oxycedrus extract [11], Anemone Coronaria extract [12], aqueous Garlic peel extract [13], Garlic extract [14], Fennel (Foeniculum Vulgare) [15], extract of Limonium Thouinii (Plumbaginaceae) [16], modified Cassava Starches [17], carboxy methylated Cassava Starch [18], Sesbania Sesban Extract [19], Rosemary extract [20], UAE Neem extract [21]. Murraya koenigii (curry leaves) extract [22], Chenopodium Ambrorsioides extract [23], Asteriscus Imbricatus extracts [24] and Argan Press Cake extract [25] have been investigated.
On the other hand, there are no studies on the use of green leafy vegetables (GLV) as inhibitors for carbon steel in acidic solutions has been published as for as use know.
Materials and Methods
Specimen preparation
Tests were performed on carbon steel specimens with the following composition (in wt. %) C 0.42%, Mn 1.23%, Si 0.265%, S 0.004%, P 0.011%, Cr 0.008%, Cu 0.024%, Sa 0.0016%, Ni 0.0214%, Ti 0.017%, Al 0.035%, Nb 0.036%, Ca 0.002% and Fe balance. Carbon steel circular strips of the same composition with an exposed area of 1 cm2 were used. Before each test, the specimen were ground with 800 and 1200 grit grinding papers, and then cleaned by distilled water and acetone.
Preparation of plant extract
GLV were dried in an electric furnace for 10 – 20 min then ground to powder. GLV dried powder (5g) were mixed with 500 ml of 1 M HCl and refluxed at 50ºC for 2 h. The extracts were cooled, and filtered through Whatman filter paper. The filtrate was then kept as the stock solution. Working solutions of different concentrations ranging from 20 to 60 % (v/v) were prepared from the stock solutions by dilution with 1 M HCl solution.
Fourier transform infrared spectroscopy (FT-IR)
A KBr pellet was made from the dried extracts and were characterized using FT-IR (Nicolet’s auxiliary experiment module – AEM, Omnic software).
Gravimetric (weight loss) method
In the gravimetric experiment, a previously weighed carbon steel specimens was completely immersed in 100 mL of 1 M HCl solution with and without the inhibitor for a period of 3 h. Then, the specimens were washed, dried and weighed. The weight loss was calculated. The experiments were repeated at different concentrations (20 – 60%), different temperatures (25°C – 60°C), and different times of immersion (3 – 15 h). From the weight loss results, corrosion rates (C.R), degree of surface coverage (θ), and the inhibition efficiency (Einh%) of the inhibitor were calculated using Eq. (1)–(3), respectively [25] and displayed in Table 1:
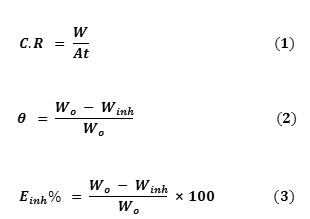
where A is the area of the carbon steel specimens (in cm2), t is the immersion time (in hours) and W is the weight loss of carbon steel after time, t, Wo and Winh are the weight losses (mg) for carbon steel.
Results and Disscusion
FTIR results of GLV extracts
The important IR absorption bands of inhibitors are given in Fig. 1 and their respective FT-IR peaks are given in Table 1. These results showed that the inhibitors containing functional groups with O and N atoms and other attached to aromatic ring, which commonly gather in corrosion inhibitors.
 |
Figure 1: FTIR spectra of GLV extracts. Click here to View figure |
Table 1 : FT-IR peaks of GLV extracts.
|
Inhibitors |
Peaks from FT-IR spectra |
Possible functional groups |
|
(A) Lactuca sativa (Lactuca) |
414.78 |
C–C (aliphatic) |
|
603.00 |
–C=C stretch |
|
|
1055.94 |
P–O–C stretch |
|
|
1245.22 |
O–SO2–O |
|
|
1407.43 |
X–SO2–X |
|
|
1614.37 |
C=N stretch |
|
|
2922.57 |
C–H (aromatic) |
|
|
3345.47 |
NH2 stretch |
|
|
3738.73 |
N–C (aromatic) |
|
|
|
||
|
(B) Eruca Sativa (Arugula) |
403.55 |
C–C (aliphatic) |
|
604.99 |
–C=C stretch |
|
|
1065.91 |
P–O–C stretch |
|
|
1247.01 |
O–SO2–O |
|
|
1414.56 |
X–SO2–X |
|
|
1623.94 |
C=N stretch |
|
|
2923.70 |
C–H (aromatic) |
|
|
3342.25 |
N–H stretch |
|
|
|
||
|
(C) Petroselinum crispum (Parsley) |
403.20 |
C–C (aliphatic) |
|
617.89 |
–C=C stretch |
|
|
1068.10 |
P–O–C stretch |
|
|
1247.46 |
O–SO2–O |
|
|
1411.10 |
X–SO2–X |
|
|
1614.22 |
C=N stretch |
|
|
2927.20 |
C–H (aromatic) |
|
|
3339.39 |
N–H stretch |
|
|
|
||
|
(D) Anethum Graveolens (Dill) |
410.82 |
C–C (aliphatic) |
|
606.26 |
–C=C stretch |
|
|
1056.64 |
P–O–C stretch |
|
|
1407.95 |
X–SO2–X |
|
|
1615.04 |
C=N stretch |
|
|
2922.35 |
C–H (aromatic) |
|
|
3348.16 |
N–H stretch |
|
|
3730.07 |
N–C (aromatic) |
|
Effect of GLV Concentration
The results indicate that the corrosion rate of carbon steel has decreased with increasing inhibitors concentration. The values of corrosion rates (C.R), and the inhibition efficiency (Einh%) obtained from the weight loss for different inhibitors concentrations at room temperature in 1 M HCl are given in Table 2. From the values obtained, it is obvious that there is a decrease in the corrosion rate of carbon steel propprtional with an increase in the concentration of GLV. This indicates that the GLV in the solution inhibits the corrosion of carbon steel in HCL and that the extent of corrosion inhibition depends on the amount of GLV present. From Fig. 2, is clear that inhibition efficiency increased with increasing the inhibitors concentration. The maximum value of inhibition efficiency (Einh%) obtained for 50% of (C) Petroselinum crispum (Parsley) is 81.39%, and the efficiency of inhibitor remained constant at concentration 60%. Due to the complex compounds or high phytochemical constituents of Petroselinum crispum (Parsley), it is difficult to assign the inhibiting action to a particular constituent or group of constituents. The inhibitor molecules present in the extracts block the surface of carbon steel via adsorption mechanism [26].
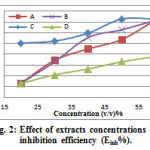 |
Figure 2: Effect of extracts concentrations on inhibition efficiency (Einh%). |
Table 2: The values of C.R, and Einh% for different inhibitors concentrations at 25ºC in 1 M HCl.
|
Inhibitors GLV |
Concentration (v/v)% |
Corrosion Rates (C.R mg/cm2.h) |
Inhibition Efficiency (Einh%) |
|
Blank |
0 |
1.86 |
– |
|
(A) Lactuca sativa (Lactuca) |
20 |
0.90 |
51.59 |
|
30 |
0.70 |
62.27 |
|
|
40 |
0.69 |
63.05 |
|
|
50 |
0.56 |
69.72 |
|
|
60 |
0.43 |
76.71 |
|
|
|
|||
|
(B) Eruca Sativa (Arugula) |
20 |
0.90 |
51.60 |
|
30 |
0.72 |
61.53 |
|
|
40 |
0.51 |
72.81 |
|
|
50 |
0.44 |
76.39 |
|
|
60 |
0.36 |
80.83 |
|
|
|
|||
|
(C) Petroselinum crispum (Parsley) |
20 |
0.55 |
70.22 |
|
30 |
0.54 |
71.15 |
|
|
40 |
0.52 |
72.08 |
|
|
50 |
0.35 |
81.39 |
|
|
60 |
0.35 |
81.39 |
|
|
|
|||
|
(D) Anethum Graveolens (Dill) |
20 |
0.90 |
51.48 |
|
30 |
0.83 |
55.53 |
|
|
40 |
0.78 |
58.22 |
|
|
50 |
0.72 |
61.57 |
|
|
60 |
0.67 |
63.93 |
|
Effect of Immersion Time
The effect of immersion time on the weight loss of carbon steel in 1 M HCl and in the presence of 50% of GLV extracts were studied at 25C. As shown in Fig. 3, it is found that weight loss of carbon steel varies with time in HCl, and was reduced in the presence of the extracts compared to the blank. The decrease in weight loss in the presence of inhibitors may be due to the adsorption of the phytochemical constituents on the surface of the carbon steel in the extract [27].
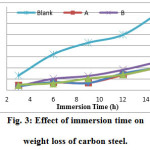 |
Figure 3: Effect of immersion time on weight loss of carbon steel. |
Effect of Temperature
The effect of temperature on the inhibition efficiency of carbon steel in 1 M HCl and in the presence of 50% of GLV extracts were studied at various temperatures (25 – 60°C) during 3 h of immersion. Fig. 4 show evolution of weight loss with absence and presence of the 50% GLV at different temperatures, and indicates that at 50% GLV, the weight loss of carbon steel has increased with temperature increase. It shows as well that inhibition efficiency has decreased at higher temperatures (Fig. 5). This is due to the increased rate of dissolution of carbon steel and partial desorption of the inhibitor from the metal surface with temperature [28, 29]. On other hand, the decrease in inhibition efficiency with increasing in temperatures reveals physical adsorption mechanism (physisorption) and may be due to increase in solubility of the protective film formed rapidly on carbon steel surface, which is believed to inhibit the process of corrosion [27].
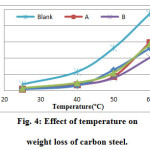 |
Figure 4: Effect of temperature on weight loss of carbon steel. Click here to View figure |
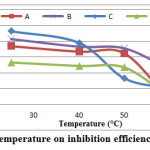 |
Figure 5: Effect of temperature on inhibition efficiency of carbon steel. |
Adsorption Isotherms and thermodynamic parameters
The mode and extent of the interaction between the GLV inhibitors and the carbon steel surfaces were studied by applying adsorption isotherms. The Langmuir, Freundlich, and Temkin approach were used to determine the adsorption mechanisms of the inhibition reaction, the isotherms were best described by the adsorption behavior of the GLV extracts on the surface of carbon steel.
Langmuir adsorption isotherm can be expressed according to Eq. 4 [30-32]:
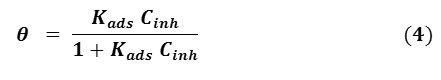
Adsorption Isotherms and thermodynamic parameters
The mode and extent of the interaction between the GLV inhibitors and the carbon steel surfaces were studied by applying adsorption isotherms. The Langmuir, Freundlich, and Temkin approach were used to determine the adsorption mechanisms of the inhibition reaction, the isotherms were best described by the adsorption behavior of the GLV extracts on the surface of carbon steel.
where Cinh is the inhibitor concentration, Kads is the adsorption equilibrium constant.
Rearranging this Eq. 4 gives Eq. 5:
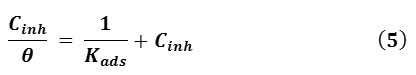
The linear variation of Cinh/θ vs. Cinh of the GLV in 1 M HCl solutions showed that the adsorption is well fitted by the Langmuir adsorption isotherm (Fig. 6 (a)), the slope and R2 presented in Table 3. The plot obeys Langmuir adsorption isotherm as the plot has linearity and good correlation coefficient (the degree of fit between the experimental data and the isotherm equation) at different exposure time. The R2 values are very close to unity, indicating strong adherence to Langmuir adsorption isotherm.
Freundlich adsorption isotherm is given by Eq. 6 [33]:

and the linearized version by Eqn. (7):
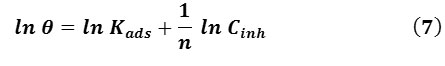
where n is the measure adsorption intensity. Values of ln θ are plotted against ln Cinh (Fig. 6 (b)). The slope and R2 data are presented in Table 3. From the slope of the Freundlich plot, 1/n is above 1, which indicates that it is cooperative adsorption.
Temkin adsorption isotherm assumes a uniform distribution of adsorption energy that increases with the increase in the surface coverage. This is given by Eq. 8 [33, 34]:
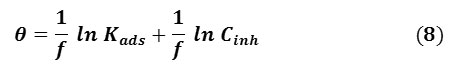
where f determines the adsorbent-adsorbates interaction. However, the plot of surface coverage θ against the natural logarithm of the concentration of the extract, ln Cinh, is shown in Fig. 6 (c), the slope and R2 are presented in Table 3.
From the Langumir, Freundlich and Temkin isotherms in Table 3, the degrees of linearity R2 were also close to unity indicating that the GLV extracts were strongly adsorbed of on the surface of the carbon steel.
From the intercept of straight lines, Kads values can be obtained and related to the free energy of adsorption, ∆Gads by Eq. 9 [35]:
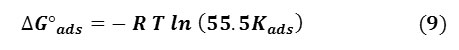
where R is the universal gas constant, T is the absolute temperature and 55.5 is the molar heat of adsorption of water. Results obtained indicate that the values of ΔG°ads are negative in all cases, showing that the reaction is spontaneous [27] and that the GLV extracts are strongly adsorbed on the carbon steel surface by physical adsorption.
Generally, the values of ΔG°ads of up to -20 kJ/mol are consistent with electrostatic interaction between charged inhibitor molecules and the carbon steel surface (physical adsorption) and those more than -40 kJ/mol involves charge sharing or transfer from the inhibitor molecule to carbon steel surface to form a coordinate bond (chemisorption). In the present study, the values of the ΔG values which are less negative than -20 kJ/mol for various inhibitors concentrations (Table 3) revealed the adsorption of the inhibitor on the metal surface confirms a physical adsorption mechanism [32, 36, 37].
The Langmuir, Freundlich, and Temkin isotherms being followed confirms a mixed adsorption mechanism owing to the spontaneity of the process. Thus, the GLV extract can be used to inhibit the carbon steel corrosion in 1 M HCl.
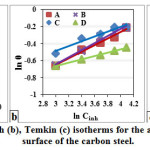 |
Figure 6: Langmuir (a), Freundlich (b), Temkin (c) isotherms for the adsorption of GLV extract on the surface of the carbon steel.Click here to View figure |
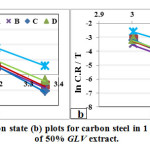 |
Figure 7: Arrhenius (a), Transition state (b) plots for carbon steel in 1 M HCl at absence and presence of 50% GLV extract.Click here to View figure |
Table 3: Langmuir, Freundlich and Temkin adsorption isotherm parameters for adsorption of GLV extract on the surface of the carbon steel.
|
Isotherm |
Langmuir |
|
Freundlich |
|
Temkin |
|||||||||
|
Inhibitor |
A |
B |
C |
D |
A |
B |
C |
D |
A |
B |
C |
D |
||
|
Slope |
0.922 |
0.876 |
0.977 |
1.372 |
0.385 |
0.418 |
0.287 |
0.197 |
0.249 |
0.273 |
0.203 |
0.113 |
||
|
Intercept |
21.107 |
21.373 |
12.966 |
12.457 |
-1.806 |
-1.903 |
-1.352 |
-1.256 |
-0.231 |
-0.301 |
0.003 |
0.174 |
||
|
R2 |
0.985 |
0.995 |
0.996 |
0.998 |
0.984 |
0.981 |
0.951 |
0.996 |
0.975 |
0.987 |
0.962 |
0.991 |
||
|
Kads |
0.0474 |
0.0468 |
0.0771 |
0.0803 |
0.164 |
0.149 |
0.259 |
0.285 |
0.396 |
0.332 |
1.015 |
4.664 |
||
|
-∆G°ads (KJ/mol) |
2.396 |
2.365 |
3.602 |
3.702 |
5.476 |
5.236 |
6.604 |
6.839 |
7.653 |
7.219 |
9.988 |
13.766 |
||
The activation energy can be calculated from Eq. 10:
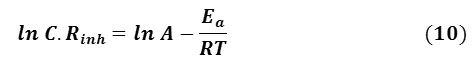
From Fig. 8 (a) the slope -Ea / R was obtained by plotting the lnicor of 1 M HCl in the absence and presence of 50% GLV versus 1/T. The calculated activation energies, Ea, are listed in Table 4. There are three groups of Ea value according to the temperature effects on the inhibition efficiency [28];
- Einh% decreases with increase in temperature, Ea (inhibited solution) > Ea (uninhibited solution).
- Einh% increases with increase in temperature, Ea (inhibited solution) < Ea (uninhibited solution).
- Einh% does not change with temperature, Ea (inhibited solution) = Ea (uninhibited solution).
It is clear from Table 4, The value of the apparent activation energy (Ea) in the presence of an inhibitor is larger than that in the blank solution, that indicate adsorption of the inhibitor was physically adsorbed on the metal surface, and that agrees case 2. Higher values of Ea in the presence of inhibitor signify increases thickness of the double layer which enhances the Ea of the corrosion process [38], and a strong inhibitory action of GLV extracts.
The enthalpy of activation (ΔH°ads) and the entropy of activation (ΔS°ads) for the corrosion of carbon steel in 1 M H Cl solution in the absence and presence of GLV were calculated from the Arrhenius equation:
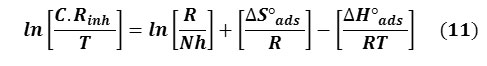
where h is the Plank’s constant (6.626176×10-34Js) and N the Avogadro’s number (6.02252×1023mol-1). A plot of lnC.Rinh/T versus 1/T gave a straight line (Fig. 8 (b)) with a slope of ΔH°ads/R and an intercept of ln(R/Nh) + ΔS°ads/R, from which the values of ΔS°ads and ΔH°ads were calculated and listed in Table 4. In both the systems, the positive signs of enthalpies ΔH°ads reflect the endothermic nature of the dissolution process.
The ΔS°ads is negative in all cases except inhibitor D with ΔS°ads value for the system without inhibitor being more negative. A negative ΔS°ads is an indication that the corrosion process is controlled by activation complex [39-41]. A closer look at the ΔS°ads values shows that the values shift towards a positive direction in the presence of the extracts. A positive shift is due to the formation of the adsorbed layer on the metal surface, which is believed to increase disorder of the system. The inhibitor layer impede the liberation of hydrogen ions at the metal surface, causing increased disorderliness resulting in increased entropy of the system [40, 42]. Also, the positive values of ΔS°ads reflect the fact that the adsorption process is accompanied by an increase in entropy, which is the leading force for the adsorption of the inhibitor onto the carbon steel surface.
Table 4: Thermodynamic activation parameters of carbon steel in 1 M HCl at absence and presence of 50% GLV extract.
|
Medium |
Blank |
A |
B |
C |
D |
|
Eads (KJ) |
60.867 |
77.171 |
73.403 |
88.270 |
69.998 |
|
∆H°ads (KJ/mol) |
58.253 |
74.557 |
70.790 |
85.651 |
67.384 |
|
∆S°ads (KJ/mol K) |
-44.312 |
-1.088 |
-14.598 |
33.282 |
-22.180 |
Conclusion
The following conclusions may be drawn from this study:
- The results obtained from the gravimetric (weight loss) method demonstrated that the GLV extracts acts as an effective inhibitor of Carbon steel corrosion in 1 M HCl, and the Petroselinum crispum (Parsley) represents the best inhibitors.
- Inhibition efficiency increases with the increase in the concentration of GLV, but decreases with immersion time, and rise in temperature.
- The adsorption of GLV on the carbon steel surface from 1 M HCl follows the Langmuir, Freundlich, and Temkin adsorption isotherms.
- The calculated values of ΔG°ads, Ea, ΔH°ads, and ΔS°ads revealed that the adsorption process are spontaneous and endothermic, and the inhibitor molecules were adsorbed on the metal surface through physical adsorption mechanism .
- Consequently, all the results show that the GLV extracts can act as an inhibitor against the corrosion of carbon steel in the HCl medium.
Acknowledgment
The researchers would like to thank the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University for providing the funding for this study under project number ج ك د/39846.
References
- Atanda, P. O.; Olorunniwo, O. E.; Alabi, O. D.; Oluwole, O. O. International Journal of Materials and Chemistry 2012, 2, 65-71.
- Sangeetha, M.; Rajendran, S.; Sathiyabama, J.; Prabhakar, P. J. Nat. Prod. Plant Resour 2012, 2, 601-610.
- da Rocha, J.; da Cunha Ponciano Gomes, J. A.; D’Elia, E. Corrosion Science 2010, 52, 2341-2348.
- Sahayaraj, J.; Amalraj, A. J.; Rajendran, S.; Muthumegala, T. S. Zastita Materijala 2010, 51, 231-236.
- Abdulkhaleq, L. Journal of Engineering and Development 2013, 17, 155-165.
- Cojocaru, A.; Maior, I.; Vaireanu, D. I.; Lingvay, C.; Lingvay, I.; Caprarescui, S.; Badea, G. E. Journal of sustaninable energy 2010, 1, 64-71.
- Nnanna, L.; Owate, I.; Nwadiuko, O.; Ekekwe, N.; Oji, W. International Journal of Materials and Chemistry 2013, 3, 10-16.
- Li, L.; Zhang, X.; Lei, J.; He, J.; Zhang, S.; Pan, F. Corrosion Science 2012, 63, 82-90.
- Kumar, K.; Pillai, M.; Thusnavis, G. Journal of Materials Science and Technology 2011, 27, 1143-1149.
- Fouda, A.; Badr, A. Afr. J. Pure Appl. Chem. 2013, 7, 350-359.
- Belkhaouda, M.; Bammou, L.; Salghi, R.; Zarrouk, A.; Hmamou, D.; Zarrok, H.; Assouag, M.; Hammouti, B.; Al-Deyab, S. Der. Pharm. Lett. 2013a, 5, 143-152.
- Belkhaouda, M.; Bammou, L.; Salghi, R.; Zarrouk, A.; Zarrok, H.; Assouag, M.; Al-Deyab, S.; Hammouti, B. Der. Pharm. Lett. 2013b, 5, 297-303.
- Assuncao, S.; Pereira, A.; Pegas, M. M.; Fernandez, T. L.; Magalhaes, M.; Schontag, T. G.; Lago, D. C.; Senna, L. F.; D’Elia, E. Corros. Sci. 2012, 65, 360-366.
- Shyamala, B.; Devi; Rajendran, S. International Journal of Chemical Science and Technology 2011, 1, 79-87.
- Lahhit, N.; Bouyanzer, A.; Desjobert, J.; Hammouti, B.; Salghi, R.; Costa, J.; Jama, C.; Bentiss, F.; Majidi, L. Portugaliae Electrochimica Acta 2011, 29, 127-138.
- Benahmed, M.; Lafhal, M.; Djeddi, N.; Laouer, H.; Akkal, S. Adv. Environ. Biol. 2012, 6, 4052-4056.
- Bello, M.; Ochoa, N.; Balsamo, V.; López-Carrasquero, F.; Coll, S.; Monsalve, A. An electrochemical and Morphological approach. Carbohydrate Polymers 2010, 82, 561-8.
- Bello, M.; Sancristóbal, J.; Ochoa, N.; Balsamo, V.; Proceedins of the 68th Annual Technical Conference and Exhibition 2010, 115.
- Al-Sahlanee, H.; Sultan, A.; Al-Faize, M. Aquatic Science and Technology 2013, 1, 135-151.
- Hassan S.; Edrah, S. Journal of Industrial Research and Technology 2011, 1, 110-113.
- Nahle, A.; Abu-Abdoun, I.; Abdel-Rahman I.; Al-Khayat, M. International Journal of Corrosion 2010, 1-9.
- Sharmila, A.; Prema, A.; Sahayaraj, P. Rasayan Journal of Chemistry 2010, 3, 74-81.
- Belkhaouda, Bammou1, M.; L.; Zarrouk, A.; Salghi, R.; Ebenso, E. E.; Zarrok, H.; Hammouti, B.; Bazzi, L.; Warad, I. Int. J. Electrochem. Sci. 2013, 8, 6033-6046.
- Senhaji, B.; Ben Hmamou, D.; Salghi, R.; Zarrouk, A.; Chebli, B.; Zarrok, H.; Warad, I.; Hammouti B.; Al-Deyab, S. Int. J. Electrochem. Sci. 2013, 8, 6033-6046.
- Adejoro, A.; Ojo, F. K.; Obafemi, S. K. Journal of Taibah University for Science 2015, 9, 196–202.
- Nnanna, L. A.; Uchendu, K. O.; Nwosu, F. O.; Ihekoronye, U.; Eti, E. P. International Journal of Materials and Chemistry 2014, 4, 34-39.
- Olasehinde, E. F., Ogunjobi, J. K.; Akinlosotu, O. M.; Omogbehin, S. A. Journal of American Science 2015, 11, 32-39.
- Nnanna, L. A.; Owate, I. O.; Oguzie, E. E. International Journal of Materials Engineering 2014, 4, 171-179.
- Singh, A.; Singh, V. K.; Quraishi, M. A. Arab J Sci Eng 2013, 38, 85–97.
- Lecante, A.; Robert, F.; Blandinieres, P. A.; Roos, C. Current Applied Physics 2011, 11, 714-724.
- Li, L.; Zhang, X.; Lei, J.; He, J.; Zhang, S.; Pan, F. Corrosion Science 2012, 63, 82–90.
- Fouda, A. S.; Etaiw, S. H.; Elnggar, W. Int. J. Electrochem. Sci. 2014, 9, 4866-4883.
- Nnanna, L. A.; Uchendu, K. O.; Nwosu, F.O.; Ihekoronye, U.; Eti, E. P. International Journal of Materials and Chemistry 2014, 4, 34-39.
- Nnanna, L. A.; Owate, I. O.; Oguzie, E. E. International Journal of Materials and Chemistry 2014, 4, 171-179.
- El Bribri, A.; Tabyaoui, M.; Tabyaoui, B.; El Attari, H.; Bentiss, F. Materials Chemistry and Physics 2013, 141, 240-247.
- Hamdy, A.; El-Gendy, N. Sh. Egyptian Journal of Petroleum 2013, 22, 17–25.
- AL-Mhyaw, S. R. Oriental Journal of Chemistry, 2014, 30, 541-552.
- Zarrok, H.; Zarrouk, A.; Salghi, R.; Assouag, M.; Hammouti, B.; Oudda, H.; Boukhris, S.; Al Deyab, S. S.; Warad, I. Der Pharmacia Lettre 2013, 5, 43-53.
- Majeed, M. H.; Sultan, A. A.; Al-Sahlanee, H. H. J. Chem. Pharm. Res. 2014, 6, 996-1001.
- Zarrok, H.; A., Salghi, R.; Assouag, M.; Hammouti, B.; Oudda, H.; Boukhris, S.; Al Deyab, S. S.; Warad, I. Der Pharmacia Lettre 2013, 5, 43-53.
- Singh, A.; Singh, V. K.; Quraishi, M. A. Arab J Sci Eng 2013, 38, 85–97.
- Olasehinde, E. F.; Olusegun, S. J.; Adesina, A. S.; Omogbehin, S. A.; Momoh-Yahayah, H. Nat. Sci. 2013, 11, 83-90.

This work is licensed under a Creative Commons Attribution 4.0 International License.









