Furfural Hydrogenation on Alloyed Copper Catalysts With Additives of Ferrosilicium
T. K. Akilov, K. R. Toktibaeva, A. A. Sadenova, K. A. Bekzhigitova, D. Asylbekova and M. Auezov
South-Kazakhstan State university, Shymkent city, Kazakhstan, 160019 Corresponding Author Email: isa.aziza@mail.ru
DOI : http://dx.doi.org/10.13005/ojc/310482
Article Received on :
Article Accepted on :
Article Published : 09 Dec 2015
The present work is dedicated to the study of influence of ferrosilicium additives [FA*-ferroalloy containing (% mass): 46.8 Si, ~53,0 Fe, other are (C, P, S) impurities] on the activity of alloyed Gu-Al = 50-50 catalyst in the reaction of furfural hydrogenation under hydrogen pressure. Components content varied (% mass): Cu- 40...49, aluminum- 50, FA* -1.0...10.0. The catalysts were prepared from 1g alloys by leaching it with 20% of aqueous solution of caustic soda in boiling water-bath during 1 hour. The phase composition and structure of alloys were investigated by means of roentgenographic and X-ray spectrum methods.
KEYWORDS:furfural; hydrogenation of furfural; high activity and selectivity; additives of ferrosilicium
Download this article as:| Copy the following to cite this article: Akilov T. K, Toktibaeva K. R, Sadenova A. A, Bekzhigitova K. A, Asylbekova D, Auezov M. Furfural Hydrogenation on Alloyed Copper Catalysts With Additives of Ferrosilicium. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Akilov T. K, Toktibaeva K. R, Sadenova A. A, Bekzhigitova K. A, Asylbekova D, Auezov M. Furfural Hydrogenation on Alloyed Copper Catalysts With Additives of Ferrosilicium. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=13174 |
Introduction
The results of numerous investigations that we conducted before [1-4], showed that ferroalloys-semi products of metallurgy plants may well be used as modifiers to increase the activity of alloyed copper catalysts, furfural hydrogenation to furfuryl alcohol (FA).
The present work is a continuation of previous investigations and is dedicated to the study of influence of ferrosilicium additives [FA*-ferroalloy containing (% mass): 46.8 Si, ~53,0 Fe, other are (C, P, S) impurities] on the activity of alloyed Gu-Al = 50-50 catalyst in the reaction of furfural hydrogenation under hydrogen pressure.
Original alloys were prepared by known technology [5]. Components content varied (% mass): Cu- 40…49, aluminum- 50, FA* -1.0…10.0. The catalysts were prepared from 1g alloys by leaching it with 20% of aqueous solution of caustic soda in boiling water-bath during 1 hour. The phase composition and structure of alloys were investigated by means of roentgenographic and X-ray spectrum methods [4].
Materials and Methods
Experiments were carried out in the autoclave of Wisniewski with capacity of 250cm3 in intensive mixing [5]. For hydrogenation 200 cm3 of 10% aqueous solution of fresh-distilled furfural and 0.5g of the leached catalyst were used. The temperature of experiment is 40…120°C, hydrogen pressure is 4…12 MPa. Hydrogenation products were analyzed in chromatograph “Chrom-4” with flame ionization detector. The activities of suspended catalysts were assessed by (W) rate of hydrogenation, expressed in g of FA 1g of catalyst for 1 hour (g/g-h). The optimum composition of the catalysts was tested in a stationary state for continuous hydrogenation of (solvent-free) furfural on enlarged laboratory installment of column type [6]. The first leaching of alloy (for 30% on Al) was conducted out-of-pile, but following (for 3…5%)- directly in the reactor with 10%-rated aqueous solution of caustic soda at 120°C. Industrial catalyst GIPKh-105 was activated by restoring it in the reactor in the stream of hydrogen at 120°C during 3 hours. The activities of stationary catalysts were assessed by their value of contact loads (W), expressed in 1 liter of furfural for 1 liter of catalyzer per 1 hour, i.e. (l/(l-h) or h-1), corresponding 98…100% output of FA. During the process the temperature, H2pressure and feed rate of furfural were increasing simultaneously, following 98…100% of FA output. In decreasing of FA output to 96% the catalyzer was in regeneration.
The results of physical-chemical and adsorptive investigations of alloys and catalysts show that FA* additives in alloys form additional compound of МеА13 structure (where Me-Cu, Fe, Si), which is included in partially leached type of catalysts; pulverize crystals of skeletal copper, increase specific surface, pore volume and size of their effective radii, sorption properties by Н2, reducing the solidity of its second basic form. All this has a positive effect on the activity of target catalysts in furfural hydrogenation reactions.
In Table 1 the data on furfuryl hydrogenation at 90°С and 6 MPa in skeletal copper catalysts from alloys with additives of FA* are presented. From Table 1 it is evident that furfuryl hydrogenation in studying copper catalysts is carried out selectively to FA, output rate of which is increases by the time of process. Furfural hydrogenation rate on copper (50% Al) catalysts with FA* additives are in 23.1…26.0 more times higher than in skeleton copper (50% Al) without additives.
Table 1: Conditions: 200 cm3 of 10% furfural aqueous solution: 90°C and 6 MPa; 0.5 g of the catalyst
|
FA* additives, %mass |
FA output (in %) during the time (min.) |
W, g/g-h |
||||||
| 0 | 10 | 20 | 30 | 40 . | 50 | |||
|
Cu (50% Al) 1,0% FA* 3,0 5,0 7,0 10,0 |
-8,2
10,0 11,3 10,7 10,0 |
-23,0
28,6 32,0 31,0. 29,0 |
-31,0
41,0 46,0 45,4 43,0 |
1,046,0
51,0 56,0 55,0 53,0 |
1,855,2
60,0 65,0- 64,0 62,0 |
2,562,0
67,0 72,5 71,2 69,0 |
3.068,0
73.0 78,0 77,2 75,0 |
1,2 -27,8
29,8 31,8 31,5 30,6 |
Maximum activity is displayed by the catalysts from the alloys with 5.0…7.0 %mass of FA*, in which FA output for 60 min. of the process reaches 78.0…77.2 %.
Fig. 1 demonstrates graphic dependence of furfural hydrogenation rate from the content of FA* in copper alloys. From Fig. 1 it is clear that curve hydrogenation speed passes through a maximum, corresponding to 5% of FA* content in copper alloy.
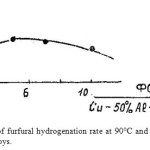 |
Figure 1: Dependence of furfural hydrogenation rate at 90°C and 6 MPa from the content of ferrosilicium in copper alloys. Click here to View figure |
Promoting effect of FA* is due to increased of pore specific surface volume and size of effective radii, sorption abilityof catalysts by H2.
The effect of hydrogen pressure and experiment temperature on the activity of skeletal Cu-5% FA(50%Al) catalyst. The results are given in Table 2. Data from Table 2 shows that with a simultaneous increase in the experimental temperature from 40 to 120°C, hydrogen pressure- in the range 4 … 12 MPafurfuryl hydrogenation rate is increased in 1.4…2.3 times. Process selectivity by FA is not violated.
Table 2: The influence of experimental temperature and hydrogen pressure on the FA formation rate with Cu-5% FA (50% Al) catalyst.
|
t,°С
|
РН2MPa
|
FA output (%) during the time (min.) |
w, g/gh |
C med., h-1
|
Reaction order |
||||
| 10 | 20 | 40 | 60 | by Н2 | PFF | ||||
| 40 | 4 | 4,6 | 7,5 | 18,5 | 33,5 | 13,7 | 5,0356 | 0,7 | 0,0 |
| 40 | 12 | 34,0 | 47,5 | 65,4 | 78,0 | 31,8 | 5,0356 | 0,7 | 0,0 |
| 60 | 4 | 8,4 | 15,6 | 28,0 | 41,5 | 16,9 | 7,7160 | 0,6 | 0,0 |
| 60 | 12 | 37,4 | 50,5 | 70,0 | 83,0 | 33,9 | 7,7160 | 0,6 | 0,0. |
| 90 | 4 | 15,0 | 26,0 | 43,5 | 57,6 | 23,5 | 11,5678 | 0,5 | 0,0 |
| 90 | 12 | 45,0 | 61,7 | 83,5 | 96,4 | 39,4 | 11,5678 | 0,5 | 0,0 |
| 100 | 4 | 25,4 | 43,0 | 60,8 | 71,4 | 29,1 | 15,4052 | 0,4 | 0,0 |
| 100 | 12 | 47,0 | 64,0 | 85,5 | 98,0 | 40,0 | 15,4052 | 0,4 | 0,0 |
| 120 | 4 | 37,0 | 51,0 | 69,8 | 83,0 | 33,9 | 21,8313 | 0,3 | 0,0 |
| 120 | 12 | 52,0 | 71,5 | 93,8 | 100,0 | 40,8* | 21,8313 | 0,3 | 0,0 |
Note: * – hydrogenation rate calculated by FA output for 50 min. of process
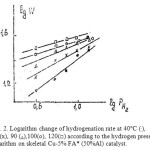 |
Figure 2: Logarithm change of hydrogenation rate at 40°C (.), 60 (x), 90 (Δ),100(о), 120(□) according to the hydrogen pressure logarithm on skeletal Cu-5% FA* (50%Al) catalyst. Click here to View figure |
Fig. 2 demonstrates the logarithmic dependence of the furfural hydrogenation rate at different temperatures from the hydrogen pressure logarithm in skeletal Cu-5% FA* (50%Al) catalyst. Fig. 2 shows that this dependence is characterized by direct lines on slope ratio that identify reaction order values on hydrogen (РН2). Table 2 shows that with a simultaneous increase in the pressure and experiment temperature the reaction order on hydrogen is reduced in the range of 0.7…0.3 g that indicates the gradual saturation of the hydrogen molecules surface.
The logarithm dependence of hydrogenation rate constant from the reciprocal temperature on skeletal Cu-5% of FA* (50% Al), the catalyst is shown in Figure 3. It implies that this dependence follows the Arrhenius equation. Value of the apparent activation energy of the reaction is equal to 18.2 kJ/mol. This indicates that furfural hydrogenation is limited by activation of unsatisfied compound on surface.
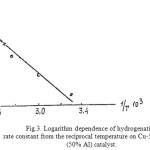 |
Figure 3: Logarithm dependence of hydrogenation rate constant from the reciprocal temperature on Cu-5% of FA* (50% Al) catalyst. Click here to View figure |
Results of the continuous (solvent-free) furfural hydrogenation under different conditions on the stationary Cu-50% Al-5% FA* catalyst are shown in Table 3.
Table 3 shows that the continuous furfural hydrogenation on alloyed stationary Cu-50% Al-5% FA* catalyst is carried out selectively to FA, output of which reaches 98…100%. Contact loads of target alloyed catalyst are 1.2…2.5 times high of corresponding value for industrial GIPKh-105 contact. With increasing the depth of aluminum leaching from alloy from 30 to 40%, the experimental temperature between 90…1400С, pressure from 2 to 8 MPa, hydrogen bubbling speed in the range of 10…180h-1 the activity of stationary C-50% Al-5% FA catalyst increases in 1.1..1.5 times. The stability of the alloyed copper catalyst in 1.8…2.6 times higher than in the industrial-GIPKh 105 contact. However, with increasing the depth of aluminum leaching from alloy the stability of alloyed catalyst decreases.
Table 3: Constant furfuryl hydrogenation on stationary Cu-50% Al-5% FA* catalyst.
|
Leaching degree of Al,% |
V catalyst cm3 |
t°С |
РН2 MPa |
WН2 h-1 |
Wff, h-1 |
Product output of reaction,% |
Approximate duration of the process, h |
|
|
FF |
FA |
|||||||
|
30 |
80,0 |
100…140 |
1.Cu-Al=50-50 4 180 0,135…0,277 |
0.4…8.0 |
92…99 |
152 |
||
|
30 35 40 |
80,0 77,5 75,0 |
80… 140 120 120 |
2. Cu-50%Al-5%FA* 4 180 0,382…0,490 2…8 180 0.4…0,5 4 10…180 0,412…0,490 |
0,0… 2,0 0,0…2,0 0,0… 1,5 |
98… 100 98…100 98,5…100 |
560 555 510 |
||
|
Recover. at 120°С during 3 hours |
90 |
90…120 |
3. Industrial GIPKh-105 4 180 0,200 |
0,0…1,5 |
85…100 |
260 |
||
Conclusion
- Ferrosilicium effect on the activity of copper alloyed (50% Al) catalyst in the reaction of periodic and continuous furfural hydrogenation under hydrogen pressure was investigated.
- It was determined that furfuryl hydrogenation on target catalysts is carried out selectively to FA, formation speed of which is 23.1…26.0 times higher than in skeletal copper (50% Al) without additives.
- Simultaneous increasing of the temperature and Н2 pressure has a positive effect of FA formation rate with skeletal Cu-FA* (50%Al) catalyst.
- It is established that target alloyed copper catalyst with ferrosilicium additives reveals a high activity, stability and in the stationary state in the reaction of continuous (solvent-free) furfural hydrogenation in FA in a wide variation conditions of technologic parameters.
References
- Beysekov T.B. Beysekov V.E., Meldeshov A.A. Multi-component copper catalysts of furfural hydrogenation. – Kropotkin of Krasnodar valley, 1990.132
- Beysekov T.B., Meldeshov A.A., Oleynikov V.E. Hydrolytic and resin industry. 1991,.4..17…19
- Yerzhanova M.S., Beysekov T.B. Alloyed catalysts of furfural hydrogenation.-Alma-Ata: Gylym, 1992.195

This work is licensed under a Creative Commons Attribution 4.0 International License.









